Metal Displacement Reactions (Edexcel IGCSE Chemistry: Double Science)
Revision Note

Author
StewartExpertise
Chemistry Lead
Metal Displacement Reactions
- The reactivity of metals decreases going down the reactivity series.
- This means that a more reactive metal will displace a less reactive metal from its compounds
- Two examples are:
- Reacting a metal with a metal oxide (by heating)
- Reacting a metal with an aqueous solution of a metal compound
- For example it is possible to reduce copper(II) oxide by heating it with zinc.
- The reducing agent in the reaction is zinc:
Zn + CuO → ZnO + Cu
zinc + copper(II) oxide → zinc oxide + copper
Metal Oxide Displacement Table
Displacement reactions between metals & aqueous solutions of metal salts
- The reactivity between two metals can be compared using displacement reactions in salt solutions of one of the metals
- This is easily seen as the more reactive metal slowly disappears from the solution, displacing the less reactive metal
- For example, magnesium is a reactive metal and can displace copper from copper(II)sulfate solution:
Mg + CuSO4→ MgSO4 + Cu
- The blue colour of the CuSO4 solution fades as colourless magnesium sulfate solution is formed
- Copper coats the surface of the magnesium and also forms solid metal which falls to the bottom of the beaker

Diagram showing the colour change when magnesium displaces copper from copper(II) sulfate
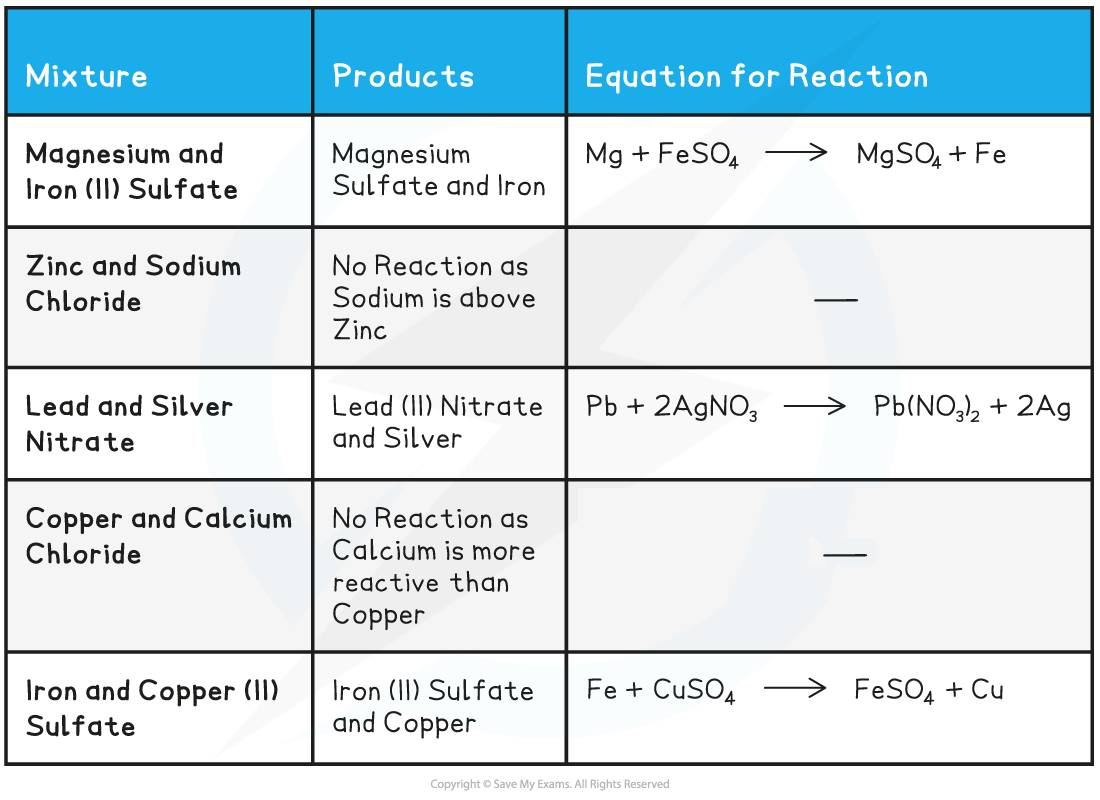
Other displacement reactions
Metal Solutions Displacement Table
Exam Tip
Displacement reactions occur when the solid metal is more reactive than the metal that is in the compound.

You've read 0 of your 0 free revision notes
Get unlimited access
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Did this page help you?