AQA GCSE Chemistry Examiner Report June 2023: Summary
Written by: Richard Boole
Reviewed by: Philippa Platt
Last updated
Contents
The June 2023 AQA GCSE Chemistry examiner reports (1F, 1H, 2F, and 2H) offer a clear window into student performance. They highlight how students responded to key topics, command words, and assessment styles across both tiers. The reports highlight widespread misconceptions, common errors, and areas where even high-attaining students underperformed.
This article distils those findings into practical, question-by-question insights for teachers. Whether you’re planning interventions, adjusting schemes of work, or preparing students for the 2025 exams, this analysis will help you focus your teaching. It shows how to turn examiner feedback into real classroom impact.
Paper 1F | Question Paper (opens in a new tab) | Mark Scheme (opens in a new tab) | Examiner's report (opens in a new tab) |
Paper 2F | Question Paper (opens in a new tab) | Mark Scheme (opens in a new tab) | Examiner's report (opens in a new tab) |
Paper 1H | Question Paper (opens in a new tab) | Mark Scheme (opens in a new tab) | Examiner's report (opens in a new tab) |
Paper 2H | Question Paper (opens in a new tab) | Mark Scheme (opens in a new tab) | Examiner's report (opens in a new tab) |
Paper 1: Themes from Foundation and Higher Tiers
The June 2023 examiner reports for AQA GCSE Chemistry Paper 1 (1F and 1H) reveal persistent weaknesses in:
Foundational concepts
Scientific terminology
Extended explanations.
Many students demonstrated good recall of basic facts. However, the step up to application, method writing, and structured reasoning caused widespread difficulty, particularly at the Higher tier. Below are six key themes from across both tiers, each with practical classroom strategies to address them.
Theme 1: Uncertainty with Atomic Structure and Subatomic Particles
Students often struggled to recall and apply core facts about protons, neutrons, and electrons.
Many Foundation-tier students confused neutron charge, despite being asked directly.
Diagrams of atomic structure showed frequent errors in particle placement or key misuse.

Correctly labelled example of an atom
Subatomic particle functions were confused or mismatched within the atom.
For example, in 1F Question 1.2, fewer than half of students correctly stated that neutrons have no charge. Many confused neutrons with electrons or protons, revealing weak recall of particle identity.
Teaching strategies:
Use particle flashcards to reinforce mass, charge, and location.
Scaffold Bohr model diagrams with drag-and-drop or labelling tasks.
Rehearse key phrasing: “neutron = neutral”, “protons and neutrons in the nucleus”.
Theme 2: Insecure Use of Terminology in Observations and Methods
Across multiple topics, students gave vague or incorrect responses when asked for observations or reasons.
“Gas given off” was commonly stated instead of “bubbles” or “effervescence”.
Some students described what was happening (e.g. “it gets hotter”) rather than what was observed.
In practical planning questions, many defaulted to listing apparatus or missed steps like filtering or crystallisation.
Teaching strategies:
Use sentence starters for observations: “I saw...”, “There was a change in...”
Model “Action → Observation” sequences in practical write-ups.
Create sorting tasks for observation vs explanation vs conclusion.
Theme 3: Difficulty with Equation Writing and Chemical Formulae
Many students lost marks due to incorrect chemical formulas, ionic charges, or unbalanced equations.
Questions on bond energy, ionic formulae, or salt preparation revealed frequent errors.
Some candidates wrote correct names alongside incorrect formulas, invalidating their answers.
Balancing simple equations caused widespread difficulty, especially when coefficients were more than 2.
For example, in 1F Q8.1, students frequently misbalanced the equation Fe2O3 + CO → Fe + CO2, often writing five or six CO molecules instead of the correct three.
Teaching strategies:
Practice ion formula construction using charge-swapping grids.
Use scaffolded examples for balancing equations, with atom counting steps shown.
Reinforce that writing both name and incorrect formula will result in zero credit.
Theme 4: Extended Responses Lacking Logical Sequencing
Across both tiers, students struggled to write full, structured answers in longer questions.
Many listed properties of a substance without linking them to its function or use.
In Level-marked questions (e.g. salt preparation or metal selection), responses often missed reasoning chains or jumped between unrelated points.
Misuse of terminology (e.g. confusing density with hardness) was common at both tiers.
The extended response in 1F Question 11.1 and 1H Question 3.1 on salt preparation revealed this clearly. Many students listed steps out of order or missed key processes like filtering and controlled evaporation, making it difficult to award Level 3 marks.
Teaching strategies:
Teach the PEEL structure: Point, Evidence, Explanation, Link.
Use comparison tables to connect structure, property, and consequence.
Model high-scoring student answers using real mark schemes for critique and annotation.
Theme 5: Calculation Steps Missed or Mishandled
Calculation-based questions across bonding, reactions, and electrolysis proved highly discriminating.
Many Foundation students lost marks by omitting units or failing to convert cm3 to dm3.
At Higher tier, students often attempted complex mole or energy calculations without showing intermediate steps. This cost them method marks when their final answers were incorrect.
In atom economy and percentage yield, some confused product/reactant positioning or rounded inaccurately.
Teaching strategies:
Reinforce a “step-by-step” layout with clearly labelled units.
Practise converting volumes and masses using structured formula triangles.
Model calculator use and rounding checks during revision sessions.
Theme 6: Misconceptions in Graph Interpretation and Data Tasks
Graph-based and tabulated data questions highlighted common issues in data handling.
Some students described what happened without referencing time, scale, or change over time.
Line of best fit tasks were often mishandled — many drew dot-to-dot lines or failed to consider balance above and below.
Others used values from data tables incorrectly, quoted the wrong axes, or missed trends altogether.
Teaching strategies:
Use graph annotation tasks: trend lines, change descriptions, key points circled.
Model “Read → Compare → Conclude” steps when interpreting numerical trends.
Practise drawing lines of best fit using pencil and explaining how they reflect data patterns.
Tier Crossover: Shared Strengths and Weaknesses
Despite differences in demand, several performance trends were common to both tiers:
Gas tests, equipment recognition, and chromatography diagrams
These were familiar, but many students missed marks due to imprecise terminology.
In 1F Q2.3, students frequently selected inappropriate equipment for accurate volume measurement, such as choosing a beaker instead of a pipette to measure 25.0 cm3.
Observation-based questions
These were poorly answered across the board, especially when students gave conclusions instead of describing visible changes.
Graph and table interpretation
Examiners commented that this has improved compared to previous years, but lines of best fit and extrapolation remain areas for development.
In 1H Question 2.2, many students mishandled the line of best fit in a data analysis question. Common errors included drawing dot-to-dot lines or ignoring the scatter of points above and below the line.
Both Foundation and Higher students showed flashes of secure recall, but often failed to link that knowledge to context, method, or data.
Teachers should reinforce tier-agnostic skills such as accurate phrasing, clear diagram use, and stepwise calculation — as these consistently help students across the full ability range.
Paper 2: Themes from Foundation and Higher Tiers
Paper 2 (2F and 2H) focused on chemistry in the Earth and atmosphere, organic chemistry, analysis, and energy changes. While some students showed confidence with required practicals and formula-based questions, many struggled with:
Extended answers
Application of the Periodic Table
Interpreting unfamiliar contexts
The themes below highlight the main patterns in student performance and provide strategies to close the gaps.
Theme 1: Inconsistent Use of Scientific Language in Observations and Explanations
Students frequently lost marks by using vague or incorrect language when describing practical outcomes.
Across gas tests and displacement reactions, terms like “gas released” were used instead of visible observations like “bubbles” or “fizzing”.
Students often described what caused a change rather than the change itself (e.g. “it reacted” vs “the solution changed colour”).
In graph-based questions, students gave explanations like “it went down” without stating that temperature or mass was decreasing.
Teaching strategies:
Use a “What did you see?” vs “What do you think happened?” activity to separate observations from explanations.
Reinforce key vocabulary through gas test charts and observation matching tasks.
Practise rewriting vague statements into scientifically acceptable observations.
Theme 2: Struggles with Equilibrium, Reversible Reactions, and Energy Concepts
Many Higher-tier students struggled to explain energy transfers in reversible reactions and how equilibrium responds to changes.
Few correctly described the effect of changing temperature or changing pressure using particle or energy-level reasoning.
Misconceptions about catalysts were common, with many stating that they change equilibrium position.
Students found it difficult to explain why concentrations stay constant at dynamic equilibrium.
For example, in 2H Q9.6, many students incorrectly claimed that a catalyst changes the position of equilibrium, a misconception explicitly flagged by the examiners.
Teaching strategies:
Use reversible reaction simulations to model shifts with temperature or pressure changes.
Reinforce Le Chatelier’s Principle with visual summary tables and sentence scaffolds.
Use diagram-based tasks to explore energy profiles for forward and reverse reactions.
Theme 3: Challenges in Practical Planning and Required Practical Recall
Planning questions and practical recall tasks highlighted several areas of weakness across both tiers.
Some students listed apparatus but gave no method, or skipped crucial steps such as measuring volumes or heating.
Practicals like chromatography or gas collection setups were often misunderstood — diagrams were incomplete or incorrectly labelled.
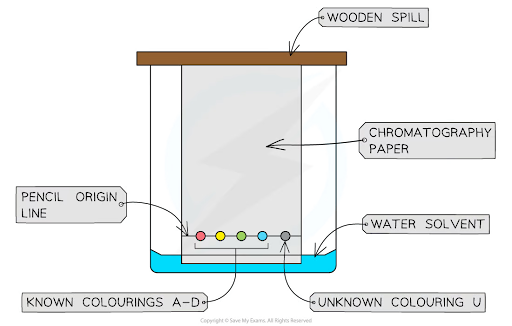
Correctly labelled example of a chromatography experiment
In energy change calculations, many students failed to rearrange formulae or apply units consistently.
For instance, in 2F Q3.1, fewer than half of students labelled a chromatography diagram correctly. Common errors included:
Placing the start line below the solvent
Misnaming the ink spot
Teaching strategies:
Use “missing method” exercises where students fill in or improve incomplete practical steps.
Reinforce practical sequences using structured flowcharts or matching cards (e.g. chromatography: pencil line → spot → solvent front).
Practice recalling required practicals from memory with visual prompts and checklists.
Theme 4: Surface-Level Interpretation of Graphs, Tables, and Equations
Although many students could quote values from data sources, few showed deeper interpretation.
In graph questions, students often missed trends or incorrectly described lines as “straight” or “flat” without quantifying changes.
Questions involving energy graphs or rate lines showed misunderstanding of sketching expectations, such as levelling off at the correct total mass lost.
Some used tables correctly to find values but failed to apply them in calculations or explanations.
Teaching strategies:
Practice “describe the pattern” and “explain the pattern” as separate steps using real graphs.
Use scaffolded sketches with prompts like “What does this line show about the rate?” or “How much gas is produced?”
Teach axis reading and data extrapolation with mark scheme phrases like “levels off at…”
Theme 5: Weaknesses in Formulae, Calculations, and Chemical Vocabulary
While some students performed well on structured questions, many struggled with basic recall and application of chemical terms and formulae.
Students confused formulae for salts, acids, and oxides, or substituted incorrect ions into equations.
Many misused vocabulary such as “ion,” “molecule,” or “compound,” often guessing unfamiliar terminology like “composite” or “alkali.”
In extended response calculations (e.g. atom economy, gas volume, percentage yield), students made early arithmetic errors or failed to follow through multi-step processes.
Teaching strategies:
Provide formula-building tasks using ion tables and the “swap and drop” method.

Example of using “swap and drop” for the formula of copper(II) chloride
Use “choose the correct term” exercises with distractors to reinforce chemical vocabulary.
Scaffold multi-step calculations using worked examples and annotation of each step.
Tier Crossover: Shared Strengths and Weaknesses
Both Foundation and Higher tier students showed similar strengths and weaknesses in key areas:
Gas test recall
This was relatively strong, but phrasing was often imprecise. The term “precipitate” was underused.
In 2F Question 8.4, many students recognised the test for hydrogen. However, they described the result vaguely, using phrases like “a sound” or “gas is released” instead of the correct “a squeaky pop with a lit splint.”
Graph interpretation
Answers to these questions have shown improvement, especially when values were clearly labelled, though many students described trends without explaining them.
In 2H Q7.4, students were asked to sketch a graph showing mass loss at a lower concentration. Many correctly drew a less steep curve but failed to level off at the appropriate value, showing misunderstanding of total gas volume changes.
Energy and equilibrium questions caused difficulty across both tiers, particularly where particle-level understanding or reversible logic was required.
Calculation questions were most successful when scaffolded, but many students failed to convert units, apply ratios, or label answers consistently.
Teachers should focus on helping all students move from surface-level recognition to deeper explanation and application — particularly when using scientific vocabulary, interpreting data, or describing how practicals work in context.
Summary: Key Lessons from Papers 1 and 2
Based on examiner feedback across the June 2023 papers, students should focus on the following areas to improve their performance in AQA GCSE Chemistry:
Be precise in your language:
Marks are often lost through vague answers. Observations must describe what is seen (e.g. “bubbles” not “gas released”), and key terms like “precipitate” or “ion” must be used correctly.Practise multi-step calculations:
Whether working out energy changes, atom economy, or concentration, students should set out each step clearly. Converting units and applying ratios are essential skills that need regular practice.Write full method sequences, not just equipment lists:
In practical planning questions, students must explain what is done and why. Marks depend on clarity, completeness, and correct sequencing of steps.Link structure to function in extended responses:
In higher-mark questions, students must go beyond listing properties. They should explain how a material’s structure or composition makes it suitable for a specific use.Read data and questions carefully:
Marks are often dropped due to misreading axes, ignoring instructions in diagrams, or copying figures without interpreting them. Encourage students to slow down, annotate, and check their working.Use required practicals to build transferable skills:
Questions on chromatography, titration, salt preparation, and energy changes appear in both theory and application formats. Students who can describe these confidently tend to perform better across both papers.
These reminders, combined with consistent use of past papers, mark scheme phrasing, and targeted practice, can make a meaningful difference to student outcomes in AQA GCSE Chemistry.
Improve Student Outcomes with Save My Exams
Save My Exams is a one-stop platform designed to boost student success in AQA GCSE Chemistry, offering a suite of revision materials and exam support. Developed by experts and trusted by teachers worldwide, our platform provides everything needed to support success in Papers 1 and 2:
Exam-Style Topic Questions with Fully Worked Solutions: Designed to reflect examiner expectations and build student confidence.
Printable Revision Notes and Diagrams: Clear, structured explanations that help students master core content and apply it in exams.
Command Word and Calculation Practice: Resources to strengthen structured reasoning and working-through in extended-response and calculation questions.
Smart Flashcards: Perfect for building retrieval strength across key definitions, facts, and formulae — whether in class or for independent revision.
Past Paper Support and Examiner Insight Integration: Helping you target revision more effectively and address real performance gaps.
Everything is designed to save you time and help your students achieve more — topic by topic, paper by paper.
Explore our AQA GCSE Chemistry Revision Resources
Join thousands of teachers already using Save My Exams to improve both confidence and grades.
Sign up for articles sent directly to your inbox
Receive news, articles and guides directly from our team of experts.

Share this article
 written revision resources that improve your
written revision resources that improve your