Line Spectra (Cambridge (CIE) A Level Physics): Revision Note
Exam code: 9702
Line spectra
Line spectra are a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours
The emitted light can be observed as a series of coloured lines with dark spaces in between
These series of coloured lines are called line or atomic spectra
Each element produces a unique set of spectral lines
No two elements emit the same set of spectral lines, therefore, elements can be identified by their line spectrum
There are two types of line spectra: emission spectra and absorption spectra
Emission spectra
When an electron transitions from a higher energy level to a lower energy level, this results in the emission of a photon
Each transition corresponds to a different wavelength of light and this corresponds to a line in the spectrum
The resulting emission spectrum contains a set of discrete wavelengths, represented by coloured lines on a black background
Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation:
Where:
ΔE = change in energy level (J)
h = Planck’s constant (J s)
f = frequency of photon (Hz)
c = the speed of light (m s-1)
λ = wavelength of the photon (m)
Therefore, this is evidence to show that electrons in atoms can only transition between discrete energy levels
Hydrogen emission spectrum

The colours on the emission spectrum refer to photons emitted from de-exciting from one level to another
Absorption spectra
An atom can be raised to an excited state by the absorption of a photon
When white light passes through a cool, low-pressure gas it is found that light of certain wavelengths are missing
This type of spectrum is called an absorption spectrum
An absorption spectrum consists of a continuous spectrum containing all the colours with dark lines at certain wavelengths
These dark lines correspond exactly to the differences in energy levels in an atom
When these electrons return to lower levels, the photons are emitted in all directions, rather than in the original direction of the white light
Therefore, some wavelengths appear to be missing
The wavelengths missing from an absorption spectrum are the same as their corresponding emission spectra of the same element
Hydrogen absorption spectrum
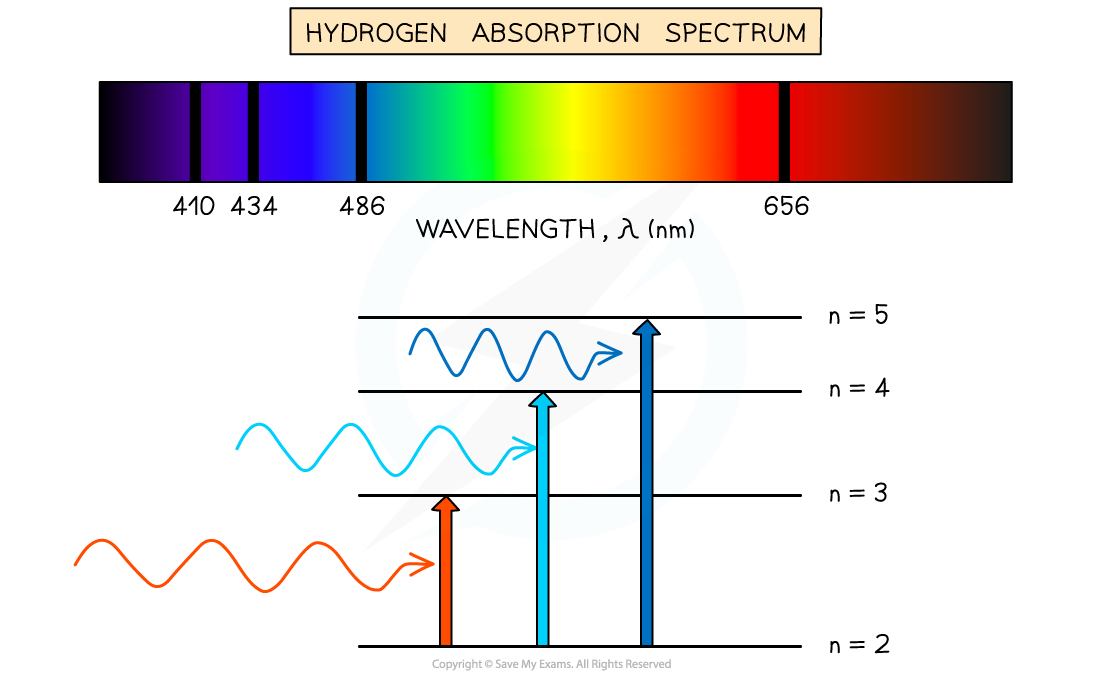
The dark lines on the absorption spectrum refer to photons absorbed from exciting from one level to another
 Unlock more revision notes. It’s free!
Unlock more revision notes. It’s free!
By signing up you agree to our Terms and Privacy Policy.
Already have an account? Log in
Did this page help you?