Balancing Chemical Equations (OCR GCSE Chemistry A (Gateway)): Revision Note
Exam code: J248
Balancing Chemical Equations
Representing Reactions as Equations
Chemical equations use the chemical symbols of each reactant and product
When balancing equations, there has to be the same number of atoms of each element on either side of the equation in accordance with the Law of Conservation of Mass
The following nonmetals must be written as molecules: H2, N2, O2, F2, Cl2, Br2 and I2 To balance an equation you work across the equation from left to right, checking one element after another
If there is a group of atoms, for example a nitrate group (NO3–) that has not changed from one side to the other, then count the whole group as one entity rather than counting the individual atoms
Examples of chemical equations:
Acid-base neutralisation reaction: NaOH (aq) + HCl (aq) ⟶ NaCl (aq) + H2O (l)
Redox reaction: 2Fe2O3 (aq) + 3C (s) ⟶ 4Fe (s) + 3CO2 (g)
In each equation there are equal numbers of each atom on either side of the reaction arrow so the equations are balanced
Balancing Equations
The best approach is to practice lot of examples of balancing equations
By trial and error change the coefficients (multipliers) in front of the formulae, one by one checking the result on the other side
Balance elements that appear on their own, last in the process
Worked Example
Example 1
Balance the following equation:
aluminium + copper(II)oxide ⟶ aluminium oxide + copper
Unbalanced symbol equation:
Al + CuO ⟶ Al2O3 + Cu
Answer

Worked Example
Example 2:
Balance the following equation:
magnesium oxide + nitric acid ⟶ magnesium nitrate + water
Unbalanced symbol equation:
MgO + HNO3 ⟶ Mg(NO3)2 + H2O
Answer

State Symbols
State symbols are written after formulae in chemical equations to show which physical state each substance is in:
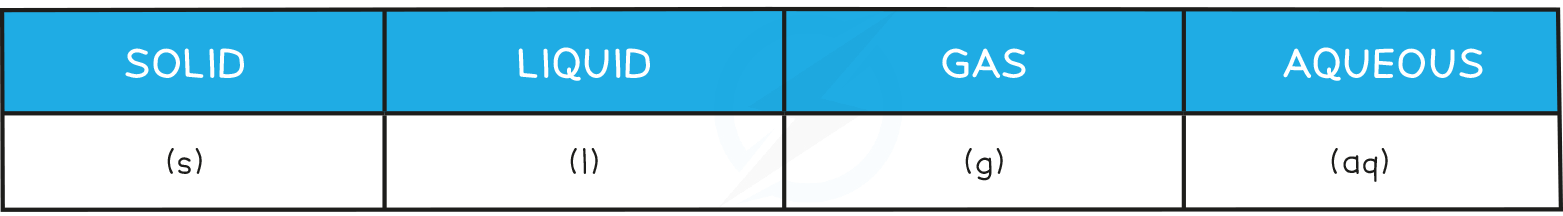
Sometimes it can be hard to know what the correct state symbol is and we have to look for clues in the identity of substances in a reaction
Generally, unless they are in a solution:
Metal compounds will always be solid, although there are a few exceptions
Ionic compounds will usually be solids
Non-metal compounds could be solids, liquids or gases, so it depends on chemical structure
Precipitates formed in solution count as solids
In the worked examples above the final equations with the state symbols would be
2Al (s) + 3CuO (s) ⟶ Al2O3 (s) + 3Cu (s)
MgO (s) + 2HNO3 (aq) ⟶ Mg(NO3)2 (aq) + H2O (l)

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?