Specific Latent Heat (OCR A Level Physics): Revision Note
Exam code: H556
Specific Latent Heat of Fusion & Vaporisation
Energy is required to change the state of substance
Examples of changes of state are:
Melting = solid to liquid
Evaporation/vaporisation/boiling = liquid to gas
Sublimation = solid to gas
Freezing = liquid to solid
Condensation = gas to liquid
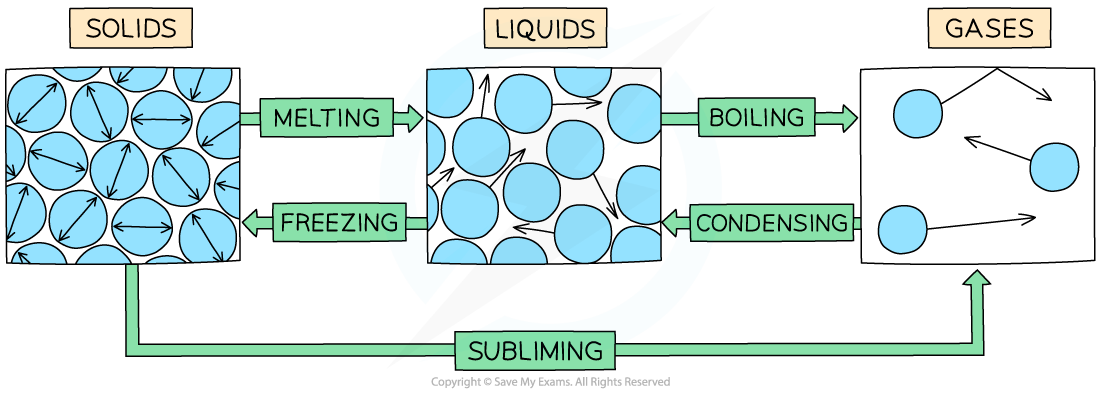
The example of changes of state between solids, liquids and gases
When a substance changes state, there is no temperature change
The energy supplied to change the state is called the latent heat and is defined as:
The thermal energy required to change the state of 1 kg of mass of a substance without any change of temperature
There are two types of latent heat:
Specific latent heat of fusion (melting)
Specific latent heat of vaporisation (boiling)
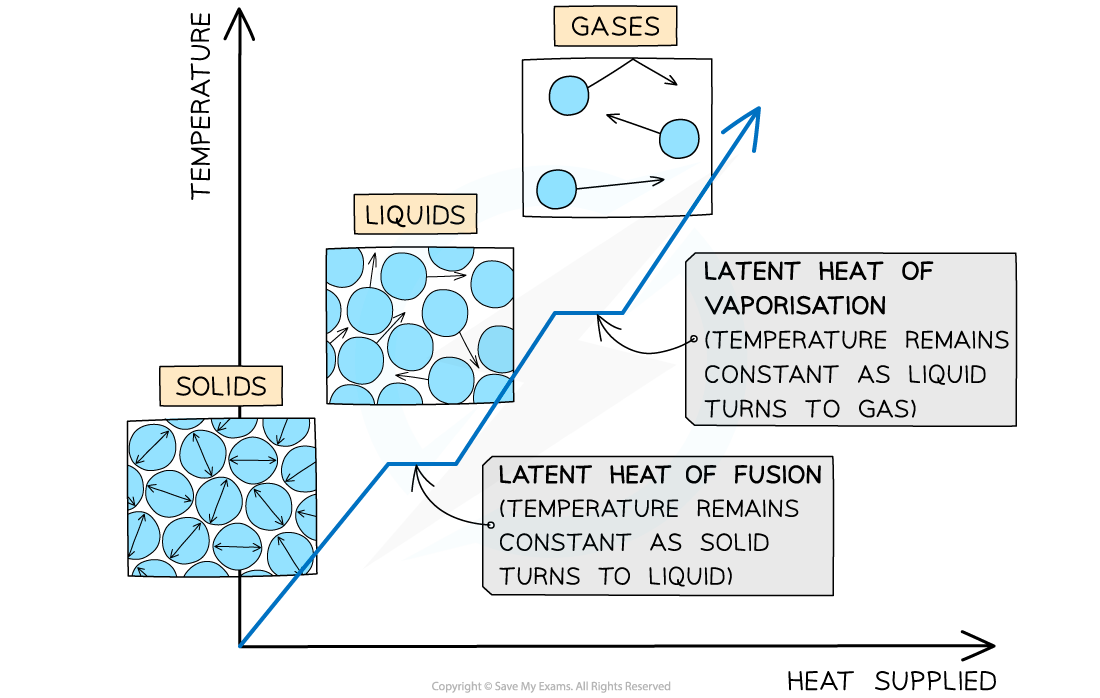
The changes of state with heat supplied against temperature. There is no change in temperature during changes of state
The specific latent heat of fusion is used when a solid is melting or a liquid is freezing
It is defined as:
The thermal energy required to convert 1 kg of solid to liquid with no change in temperature
The specific latent heat of vaporisation is used when a liquid is vapourising or a gas is condensing
It is defined as:
The thermal energy required to convert 1 kg of liquid to gas with no change in temperature
Determining Specific Latent Heat
The amount of energy E required to melt or vaporise a mass of m with latent heat L is:
E = mL
Where:
E = amount of thermal energy to change the state (J)
L = latent heat of fusion or vaporisation (J kg−1)
m = mass of the substance changing state (kg)
Specific latent heat of fusion is represented by Lf
Specific latent heat of vaporisation is represented by Lv
The values of latent heat for water are:
Specific latent heat of fusion = 330 kJ kg−1
Specific latent heat of vaporisation = 2.26 M J kg−1
Therefore, evaporating 1 kg of water requires roughly seven times more energy than melting the same amount of ice to form water
The reason for this is to do with intermolecular forces:
When ice melts: energy is required to increase the molecular separation until they can flow freely over each other
When water boils: energy is required to completely separate the molecules until there are no longer forces of attraction between the molecules,
This requires much more energy
Worked Example
The energy needed to boil a mass of 530 g of a liquid is 0.6 MJ.
Calculate the specific latent heat of the liquid and state whether it is the latent heat of vaporisation or fusion.
Answer:
Step 1: State the known values
Mass, m = 530 g = 0.53 kg
Energy supplied = 0.6 MJ = 0.6 × 106 J
Step 2: State the specific latent heat equation
E = mL
Step 3: Rearrange for latent heat
Step 4: Substitute in the values
Step 5: State whether the value is the specific latent heat of vaporisation or fusion
L is the latent heat of vaporisation because the change in state is from liquid to gas (boiling)
Examiner Tips and Tricks
Use these reminders to help you remember which type of latent heat is being referred to:
Latent heat of fusion = imagine ‘fusing’ the liquid molecules together to become a solid
Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas
Procedures to Determine Specific Latent Heat
Determining the Specific Latent Heat of Fusion, Lf
Equipment List
Crushed ice
Two funnels with filter paper
Three retort stands
Two thermometers
Two electric balances
An appropriate heater (e.g., an immersion heater)
A power source
A voltmeter, ammeter and stop-clock
Method
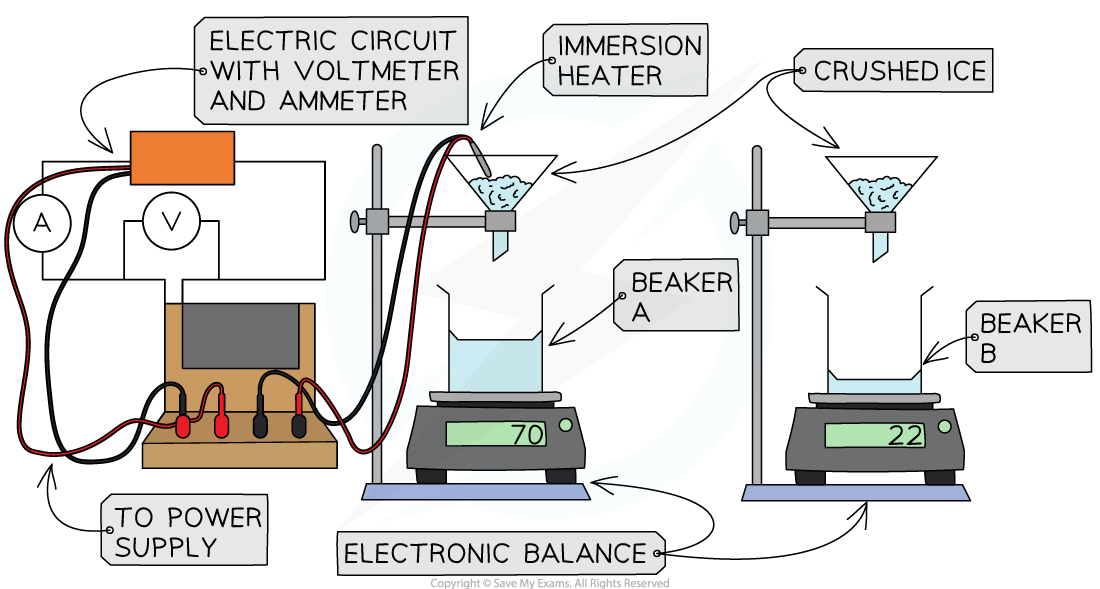
Place a beaker on each balance
Leaving the beaker on the balance, zero the scale
Arrange a funnel, clamped above each beaker
Set up an immersion heater
Connect to the power source
Add an ammeter in series and a voltmeter in parallel
Place the immersion heater in one of the funnels
Measure out 50-100g of ice
Add the same mass of ice to each beaker
Record this value
Turn on the immersion heater and start the stop watch
Record the potential difference and current
After a suitable period of time (around 5-10 minutes) remove the funnels, stop the stop watch and turn off the heater
Record the mass of water in the beaker
Analysis
The energy supplied to the ice can be calculated using the equation:
energy = current x potential difference x time
Using the values for current, potential difference and time, calculate the energy supplied
The specific latent heat of fusion can be calculated using the equation:
energy = mass x specific latent heat
The change mass is equal to the mass of water collected
To take into account melting due to heat transfer from the surroundings find the difference in mass between the two beakers of water
This gives the change in mass due to the energy supplied by the heater
Calculate the mass of the melted ice and convert it into kg
Δm = mA - mB
Mass in g ÷ 1000 = Mass in kg
Calculate the specific latent heat of fusion of ice to water using the equation for specific latent heat
Evaluation
Errors may be introduced due to precision of the instruments
Water may be absorbed by the filter paper
This will reduce the mass and therefore give a higher value for specific latent heat
Determining the Specific Latent Heat of Vaporisation, Lv
Equipment List
A double-walled glass vessel with an inner flask containing water
An appropriate electric heater (e.g., an immersion heater)
A condenser with a collecting flask
A power source
A voltmeter, ammeter and stop-clock
An electric balance
Method
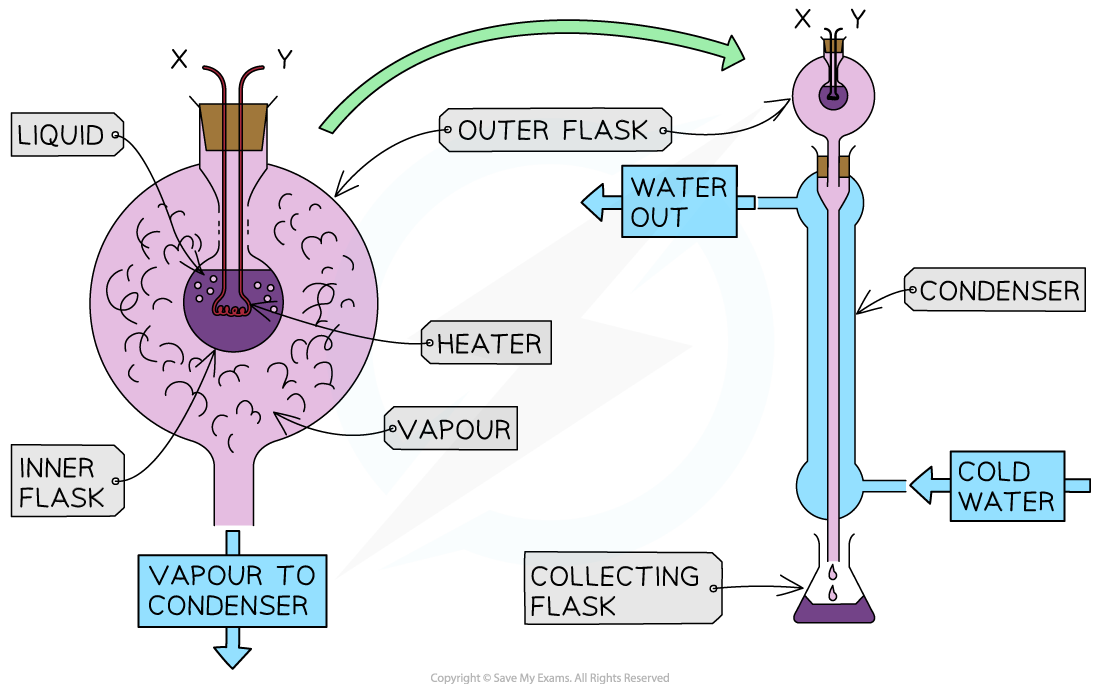
Connect the double-walled glass vessel to the condenser
Place the collecting flask at the end of the condenser
Set up an immersion heater
Connect to the power source
Add an ammeter in series and a voltmeter in parallel
Place the immersion heater in the fluid
Turn on the immersion heater and start the stop watch
Record the potential difference and current
After a suitable period of time (around 5-10 minutes), stop the stop watch and turn off the heater
Record the mass of water collected in the conical flask
Analysis
The energy supplied to the water can be calculated using the equation:
energy = current x potential difference x time
Using the values for current, potential difference and time, calculate the energy supplied
The specific latent heat of vaporisation can be calculated using
The mass of water collected
The energy supplied calculated
The equation:
energy = mass x specific latent heat
Evaluation
Errors may be introduced due to precision of the instruments
Not all of the vapour which enters the condenser may make it to the beaker

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?