Alpha, Beta & Gamma Radiation (OCR A Level Physics): Revision Note
Exam code: H556
Alpha, Beta & Gamma Particles
Some elements have nuclei that are unstable
This tends to be when the number of nucleons does not balance
In order to become more stable, they emit particles and/or electromagnetic radiation
These nuclei are said to be radioactive
There are three different types of radioactive emission: Alpha, Beta and Gamma
Alpha Particles
Alpha (α) particles are high energy particles made up of 2 protons and 2 neutrons (the same as a helium nucleus)
They are usually emitted from nuclei that are too large

Alpha is a low penetrating type of radiation
Alpha particles have a range of a few cm in air
Beta Particles
Beta (β−) particles are high energy electrons emitted from the nucleus
β− particles are emitted by nuclei that have too many neutrons
Beta is a moderately ionising type of radiation
This is due to it having a charge of +1e
This means it is able to do some slight damage to cells (less than alpha but more than gamma)
Beta is a moderately penetrating type of radiation
Beta particles have a range of around 20 cm - 3 m in air, depending on their energy
Beta can be stopped by a few millimetres of aluminium foil

Gamma Rays
Gamma (γ) rays are high energy electromagnetic waves
They are emitted by nuclei that need to lose some energy

Gamma is a highly penetrating type of radiation
Gamma particles have a range of around 1- 10 cm in lead or several metres in concrete
If these particles hit other atoms, they can knock out electrons, ionising the atom
This can cause chemical changes in materials and can damage or kill living cells
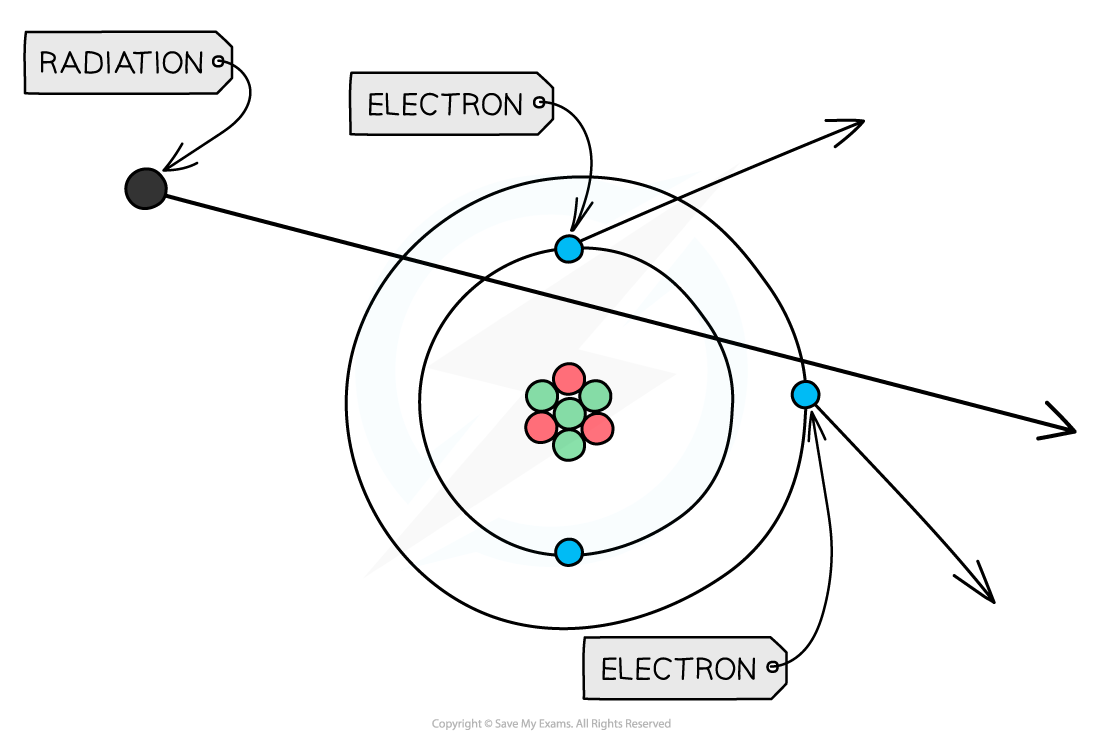
When radiation passes close to atoms, it can knock out electrons, ionising the atom
The properties of the different types of radiation are summarised in the table below

u is the atomic mass unit (see “Atomic Mass Unit (u)”)
e is the charge of the electron: 1.60 × 10-19 C
c is the speed of light: 3 × 108 m s-1
Worked Example

Answer: D

Examiner Tips and Tricks
It is important to be confident with the properties of each type of radiation and how they can be written as symbols.
Investigating the Absorption of Alpha, Beta & Gamma Radiation
Aim of the Experiment
The aim of this experiment is to investigate the penetration powers of different types of radiation using either radioactive sources or simulations
Variables:
Independent variable = Absorber material
Dependent variable = Count rate
Control variables:
Radioactive source
Distance of GM tube to source
Location / background radiation

Method

Investigating radiation apparatus
Connect the Geiger-Müller tube to the counter and, without any sources present, measure background radiation over a one minute period
Repeat this three times, and take an average
Now place a radioactive source a fixed distance of 3 cm away from the tube and take another reading over a one minute interval
Now take a set of absorbers: some paper, several different thicknesses of aluminium (increasing in 0.5mm intervals) and different thickness of lead
One at a time, place these absorbers between the source and the tube and take another reading over a one minute interval
Repeat the above experiment for other radioactive sources
Results
Alpha radiation will be absorbed by the paper
Beta radiation will be absorbed by the aluminium foil
Some gamma radiation will be absorbed by the thick lead

Penetrating power of alpha, beta and gamma radiation
Safety Considerations
When not using a source, keep it in a lead lined container
When in use, try and keep a good distance (a metre or so) between yourself and the source
When handling the source, do so using tweezers (or tongs) and point the source away from you
Examiner Tips and Tricks
It is common for you to be asked questions on this practical. Make sure you are familiar with the safety procedures and why you would measure the background radiation first before completing the experiment.
Ready to test your students on this topic?
- Create exam-aligned tests in minutes
- Differentiate easily with tiered difficulty
- Trusted for all assessment types
Did this page help you?