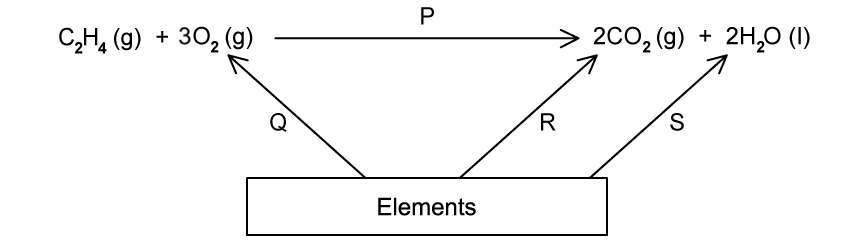
Enthalpy changes that are difficult to measure directly can often be determined using Hess’ Law to construct an enthalpy cycle.
Which enthalpy change is indicated by X in the enthalpy cycle shown?

+ 1 x Enthalpy of formation of water
- 1 x Enthalpy of formation of water
+ 3 x Enthalpy of formation of water
- 3 x Enthalpy of formation of water
Was this exam question helpful?