Half-Life (OCR GCSE Physics A (Gateway)): Revision Note
Exam code: J249
Half-Life
It is impossible to know when a particular unstable nucleus will decay
But the rate at which the activity of a sample decreases can be known
This is known as the half-life
Half-life is defined as:
The time it takes for the number of nuclei of a sample of radioactive isotopes to decrease by half
In other words, the time it takes for the activity of a sample to fall to half its original level
Different isotopes have different half-lives and half-lives can vary from a fraction of a second to billions of years in length
Using Half-life
Scientists can measure the half-lives of different isotopes accurately:
This means it would take 704 million years for the activity of a uranium-235 sample to decrease to half its original amount
Uranium-235 has a half-life of 704 million years
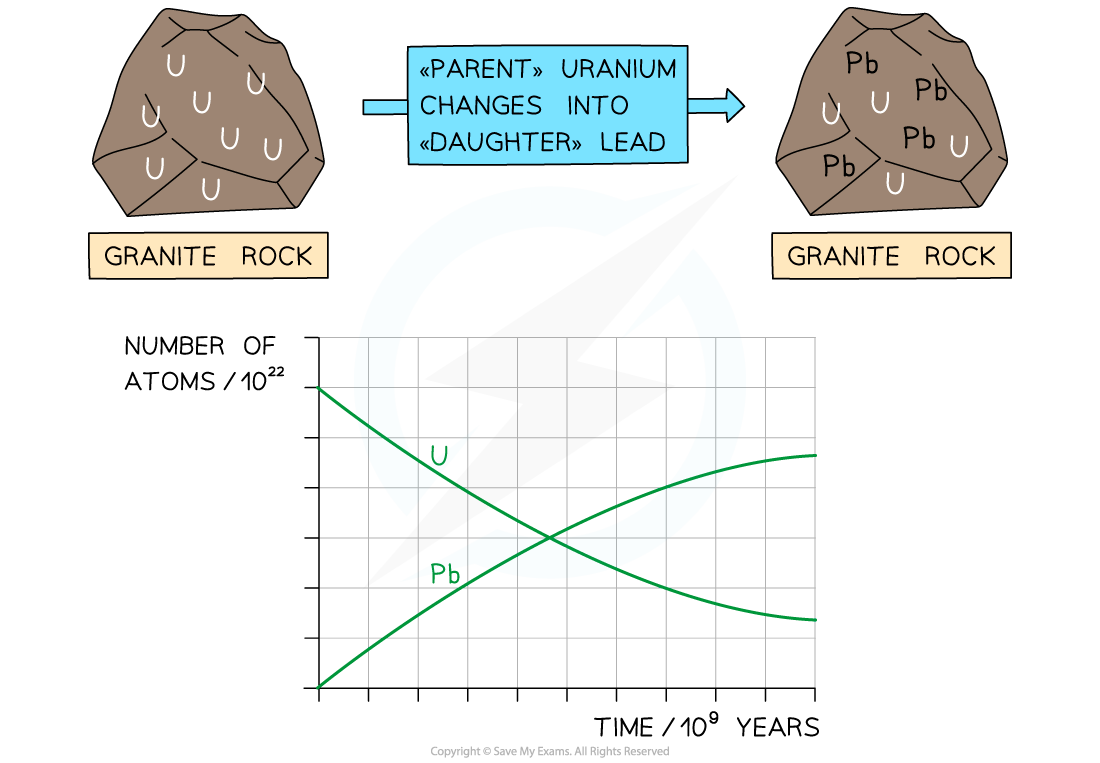
Uranium atoms decay whilst the number of lead atoms increases
Carbon-14 has a half-life of 5700 years
So after 5700 years, there would be 50% of the original amount of carbon-14 remaining
After two half-lives, or 11 400 years, there would be just 25% of the carbon-14 remaining
With each half-life, the amount remaining decreases by half

Unlock more, it's free!
Was this revision note helpful?
