Chlorine reacts with water to form chlorine water via the following equation.
Cl2 + H2O → HOCl + HCl
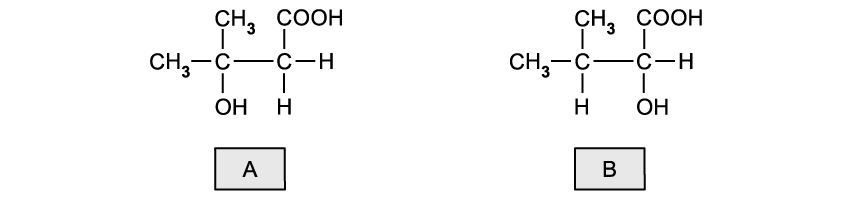
State the oxidation number of chlorine in the following species:
Cl2
HOCl
HCl
Chlorine is an oxidising agent.
Define oxidising agent in terms of electrons.
Nitrogen monoxide, NO, is formed when silver metal reduces nitrate ions, NO3- , ions in an acidic solution. State the oxidation numbers of nitrogen in NO and NO3-.
State the half equation for the formation of silver ions, Ag+ (aq), from silver metal.
Was this exam question helpful?