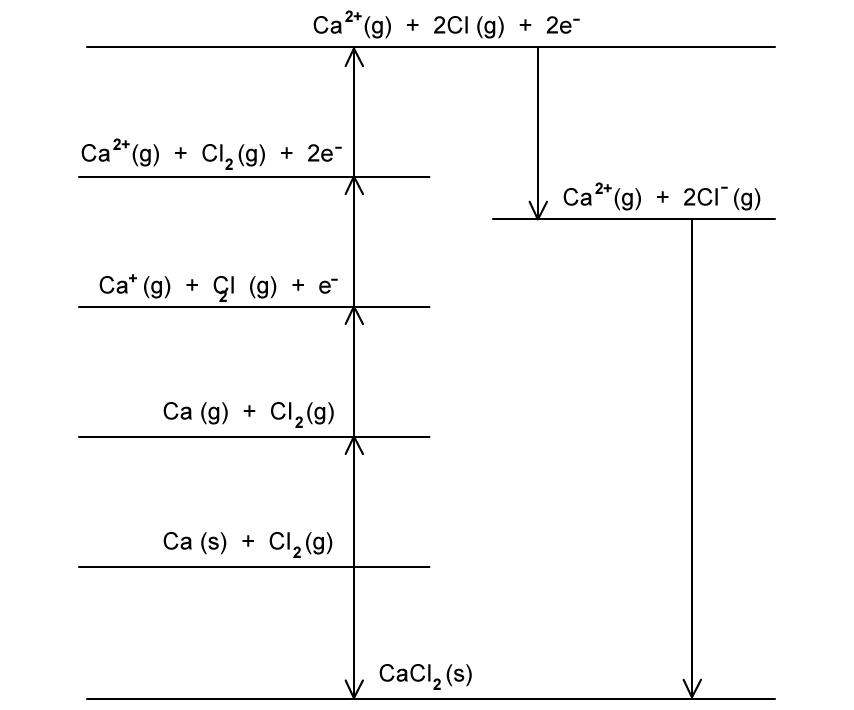
State Hess’s Law.
State the type of system in which the total amount of matter present is always constant.
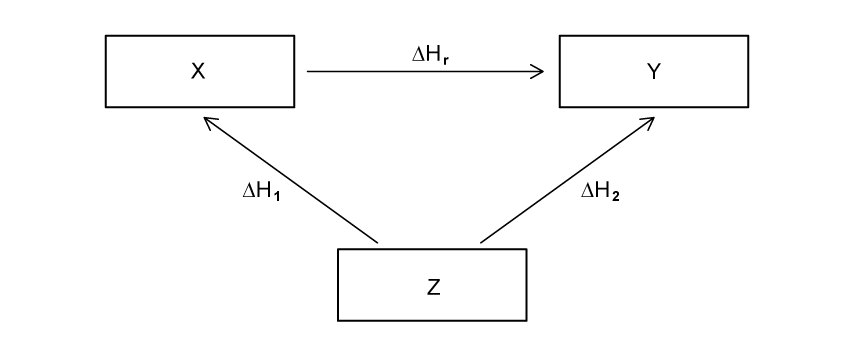
Using the image below, construct an equation that can be used to determine ΔHr from ΔH1 and ΔH2.
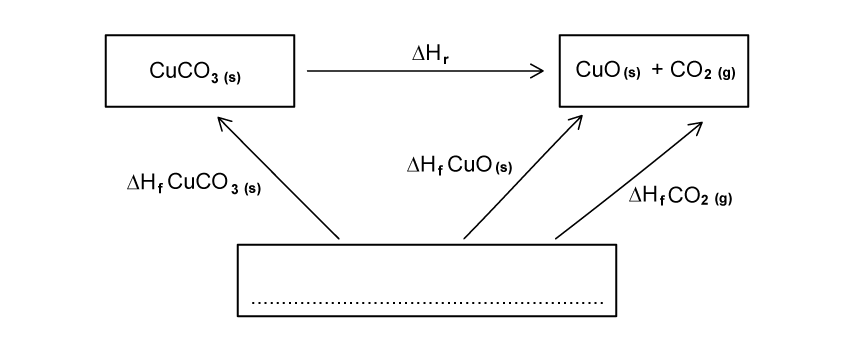
Complete the following Hess’s Law cycle for the decomposition of copper carbonate.
Was this exam question helpful?