The Ideal Gas Equation (OCR A Level Chemistry A): Revision Note
Exam code: H432
Ideal gas equation & calculations
Kinetic theory of gases
The kinetic theory of gases explains the behaviour of gas molecules, which are in constant motion
It is based on these assumptions:
Gas molecules move rapidly and randomly
The volume of individual gas molecules is negligible
There are no attractive or repulsive forces between molecules
Collisions between molecules are elastic
This means that no kinetic energy is lost
Temperature is proportional to the average kinetic energy of the molecules
Gases that follow this model exactly are called ideal gases
Real gases do not follow all these assumptions perfectly
But, they often behave similarly under standard conditions
Ideal gases
The volume that an ideal gas occupies depends on:
Pressure
Temperature
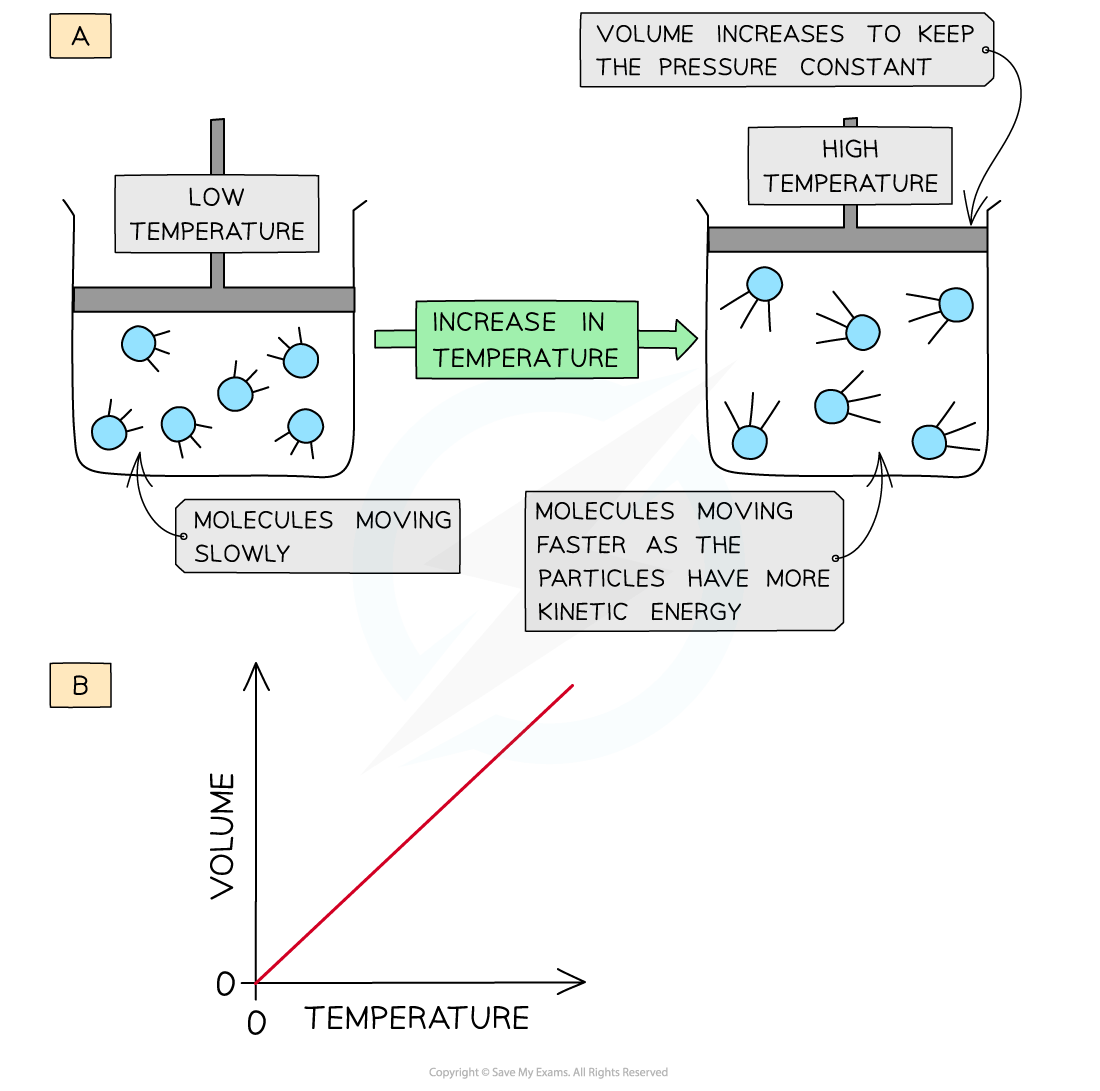
When a gas is heated at constant pressure:
Particles gain kinetic energy
They collide more frequently with the container walls
To maintain constant pressure, the particles must spread out
So, the volume increases
Therefore, volume is directly proportional to temperature at constant pressure
Limitations of the ideal gas law
At low temperatures and high pressures, real gases deviate from ideal behaviour
Molecules are closer together
Intermolecular forces (e.g. London forces, permanent dipoles) become significant
These forces reduce collisions with container walls
Therefore, the measured pressure is lower than predicted
The actual volume of gas particles is no longer negligible
Therefore, the measured volume is lower than predicted
Therefore, under these conditions:
The assumption of no attraction between particles is invalid
The assumption that particles have negligible volume is also invalid
Ideal gas equation
The ideal gas equation shows the relationship between pressure, volume, temperature, and number of moles:
pV = nRT
Where:
p = pressure (pascals, Pa)
V = volume (m3)
n = number of moles of gas (mol)
R = gas constant (8.314 J mol-1 K-1)
T = temperature (kelvin, K)
Worked Example
Calculating the volume of a gas
Calculate the volume occupied by 0.781 mol of oxygen at a pressure of 220 kPa and a temperature of 21 °C.
Answer:
Rearrange the ideal gas equation to find volume of gas
V =
Convert units:
p = 220 kPa = 220 000 Pa
n = 0.781 mol
R = 8.314 J mol-1 K-1
T = 21 oC = 294 K
Substitute and calculate V:
V =
V = 0.00867 m3
V = 8.67 dm3
Worked Example
Calculating the molar mass of a gas
A flask of volume 1000 cm3 contains 6.39 g of a gas. The pressure in the flask was 300 kPa and the temperature was 23 °C.
Calculate the relative molecular mass of the gas.
Answer
Rearrange the ideal gas equation to find the number of moles of gas:
n =
Convert units:
p = 300 kPa = 300 000 Pa
V = 1000 cm3 = 1 dm3 = 0.001 m3
R = 8.314 J mol-1 K-1
T = 23 oC = 296 K
Substitute and calculate n:
n =
n = 0.1219 mol
Use n and mass to calculate molar mass:
n =
molar mass =
molar mass = 52.42 g mol-1
Examiner Tips and Tricks
Always convert units:
Pressure to Pa
Volume to m3
Temperature to K (oC + 273)
Be confident rearranging pV = nRT to solve for any variable
Use the value of R from the Data Sheet: 8.314 J mol-1 K-1

Unlock more, it's free!
Was this revision note helpful?
