The Nitrogen Cycle (College Board AP® Environmental Science): Study Guide
What is the nitrogen cycle?
The nitrogen cycle is the set of processes by which nitrogen atoms and nitrogen-containing molecules move through different parts of the environment, including:
The atmosphere
Soil
Water
Living organisms
Nitrogen is essential for life because it is a key component of proteins and nucleic acids (DNA and RNA)
The nitrogen cycle ensures:
Nitrogen is converted into forms that plants and animals can use
Nitrogen is then recycled back into the atmosphere or soil
Nitrogen moves through sources (which release nitrogen) and sinks (which absorb nitrogen)
Nitrogen sources
Nitrogen sources release nitrogen compounds into the environment
Examples include:
Lightning strikes: convert atmospheric nitrogen into nitrates (NO₃⁻)
Decomposition of organic material: breakdown of dead plants and animals releases nitrogen as ammonium (NH₄⁺)
Fertilizer application: adds nitrogen compounds like nitrates and ammonium to the soil
Animal waste: releases ammonia and nitrates into the environment
Nitrogen sinks
Nitrogen sinks absorb or remove nitrogen from another part of the nitrogen cycle
Examples include:
Atmosphere
Soil
Plants and animals
Oceans
Nitrogen reservoirs
Nitrogen reservoirs are places where nitrogen compounds are stored within the cycle
Most reservoirs, like soil and living organisms, hold nitrogen compounds for relatively short periods (days to decades)
These are known as short-term reservoirs
Some reservoirs, like sediments in oceans and sedimentary rocks, can store nitrogen for longer periods (thousands to millions of years)
These are known as long-term reservoirs
Key nitrogen reservoirs
Atmosphere:
Contains nitrogen gas (N₂), which makes up about 78% of Earth's atmosphere
Soil:
Holds nitrogen in forms like nitrates (NO₃⁻) and ammonium (NH₄⁺), which plants can absorb
Living organisms:
Store nitrogen in organic forms such as proteins and nucleic acids
Fixation of atmospheric nitrogen
Fixation is the process that converts nitrogen gas (N₂) from the atmosphere into forms that plants can use
E.g. ammonia (NH₃)
Plants cannot directly use nitrogen gas, so fixation is a crucial step in the nitrogen cycle
Types of nitrogen fixation
Biotic fixation:
Performed by nitrogen-fixing bacteria like rhizobium (found in legume root nodules)
These bacteria use enzymes to convert nitrogen gas into ammonia
Abiotic fixation:
Occurs through natural processes such as lightning
High energy from lightning breaks nitrogen molecules
This allows the nitrogen to react with oxygen to form nitrates
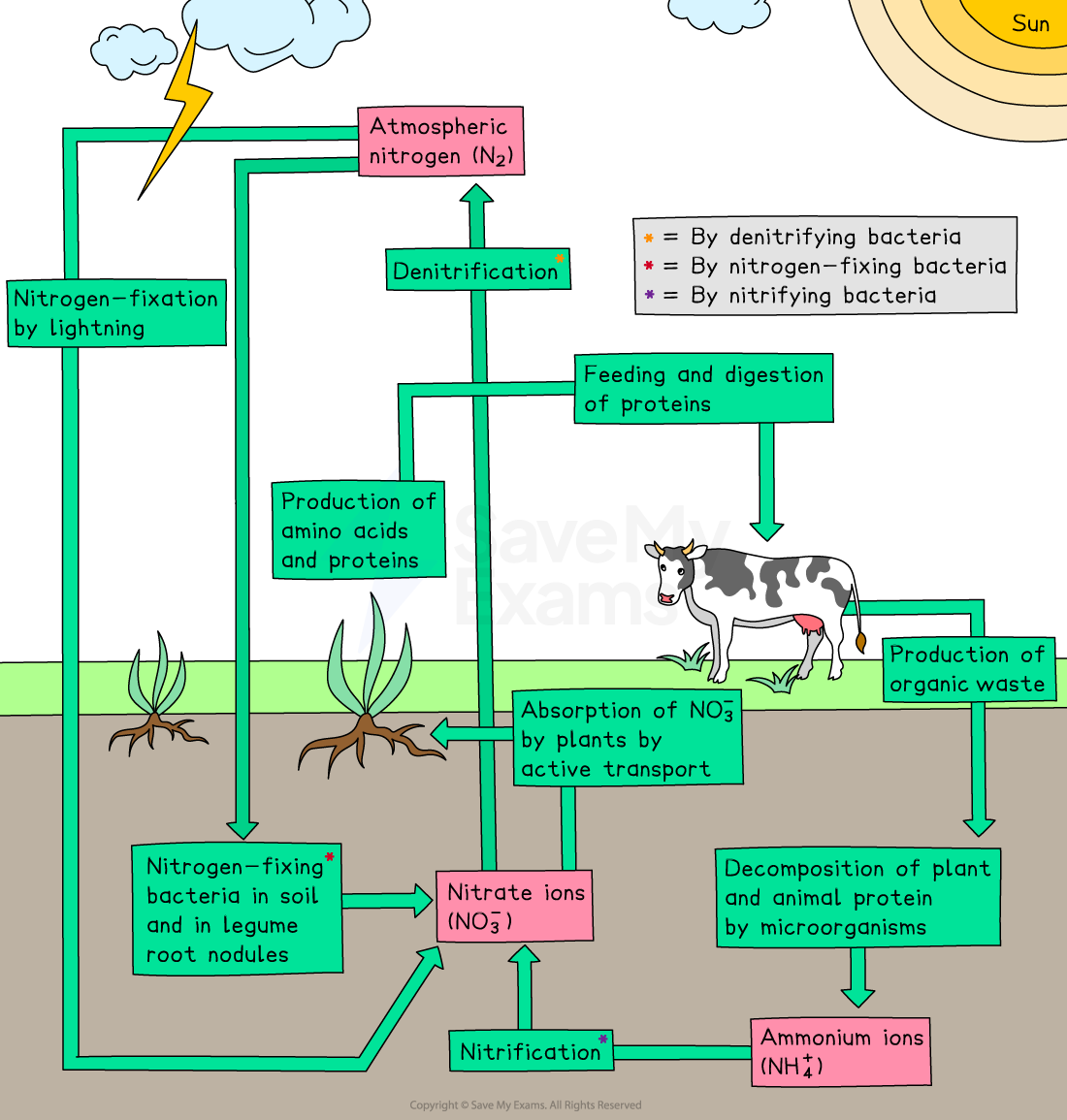
The role of bacteria in the nitrogen cycle
Bacteria are essential for converting nitrogen into usable forms
Nitrogen fixation: nitrogen-fixing bacteria (such as rhizobium) convert atmospheric nitrogen (N2) into ammonia (NH3), which plants can use
This can happen in the soil or through symbiotic relationships with plants like legumes
Nitrification: nitrifying bacteria convert ammonia (NH₃) into nitrites (NO₂⁻), then into nitrates (NO₃⁻)
Plants absorb these nitrates through their roots
Denitrification: denitrifying bacteria convert nitrates (NO₃⁻) back into nitrogen gas (N₂)
This nitrogen gas then returns to the atmosphere
This process happens in anaerobic (low oxygen) conditions, like waterlogged soils
Decomposition (ammonification): when plants and animals die, decomposing bacteria break down their nitrogenous compounds into ammonium (NH₄+)
Atmospheric nitrogen reservoir
The atmosphere is the largest nitrogen reservoir
It contains nitrogen gas in its inert form (N₂)
Although nitrogen gas is abundant, most organisms cannot use it directly
Processes like nitrogen fixation make this nitrogen available to plants and animals in usable forms like ammonia and nitrates
Denitrification returns nitrogen to the atmosphere as gas, completing the cycle
Importance of the atmospheric nitrogen reservoir
Acts as a constant source of nitrogen for ecosystems
Supports processes like fixation
This enables nitrogen to enter the biosphere
For example, after a thunderstorm:
Nitrogen from the atmosphere is converted into nitrates
Nitrates are deposited into the soil by rain
This enriches the soil for plant growth

Unlock more, it's free!
Did this page help you?