Calculating Radioactive Decay (OCR GCSE Physics A (Gateway)): Revision Note
Exam code: J249
Calculating Radioactive Decay
Higher Tier Only
To calculate the half-life of a sample, the procedure is:
Measure the initial activity, A0, of the sample
Determine the half-life of this original activity
Measure how the activity changes with time
The time taken for the activity to decrease to half its original value is the half-life
Half-life can be shown clearly on a graph
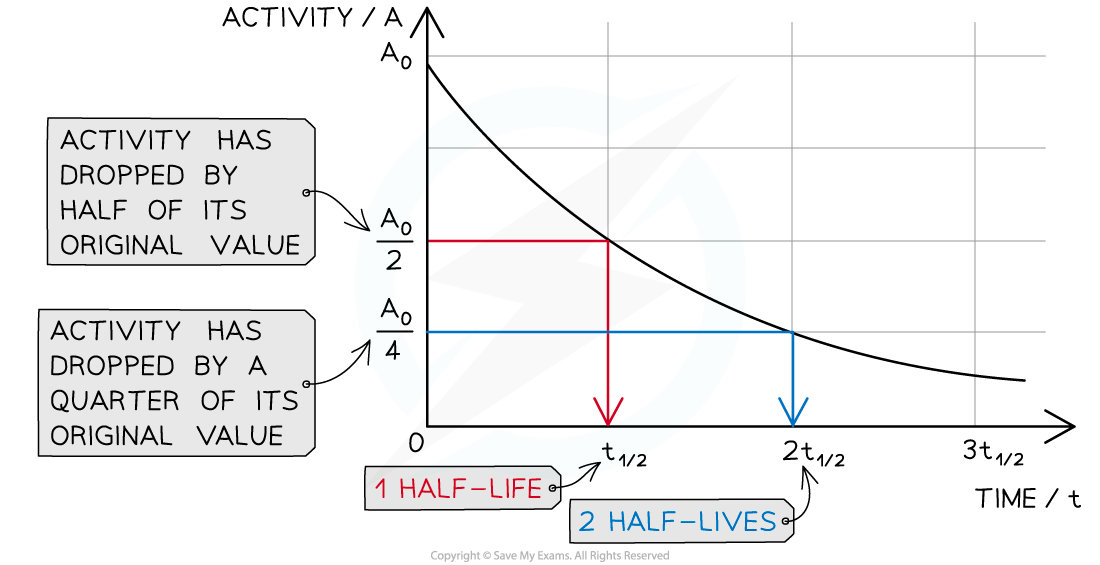
The diagram shows how the activity of a radioactive sample changes over time. Each time the original activity halves, another half-life has passed
The time it takes for the activity of the sample to decrease from 100 % to 50 % is the half-life
It is the same length of time as it would take to decrease from 50 % activity to 25 % activity
The half-life is constant for a particular isotope
Worked Example
The radioisotope technetium is used extensively in medicine. The graph below shows how the activity of a sample varies with time.
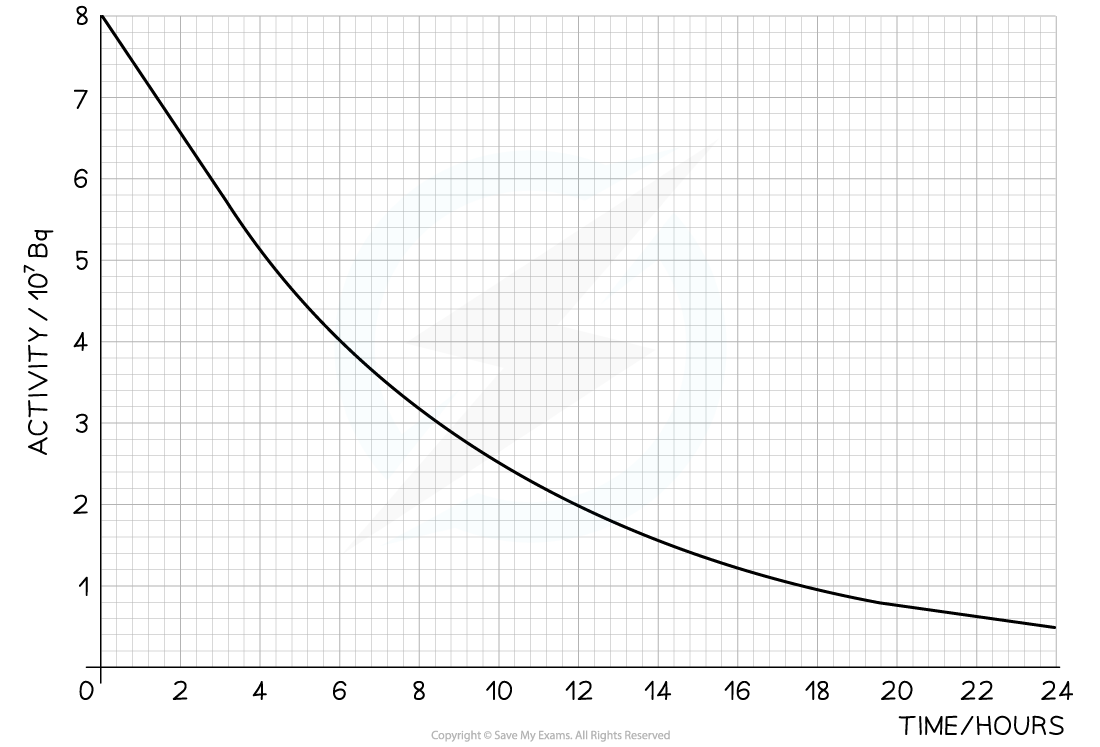
Determine the half-life of this material.
Answer:
Step 1: Draw lines on the graph to determine the time it takes for technetium to drop to half of its original activity
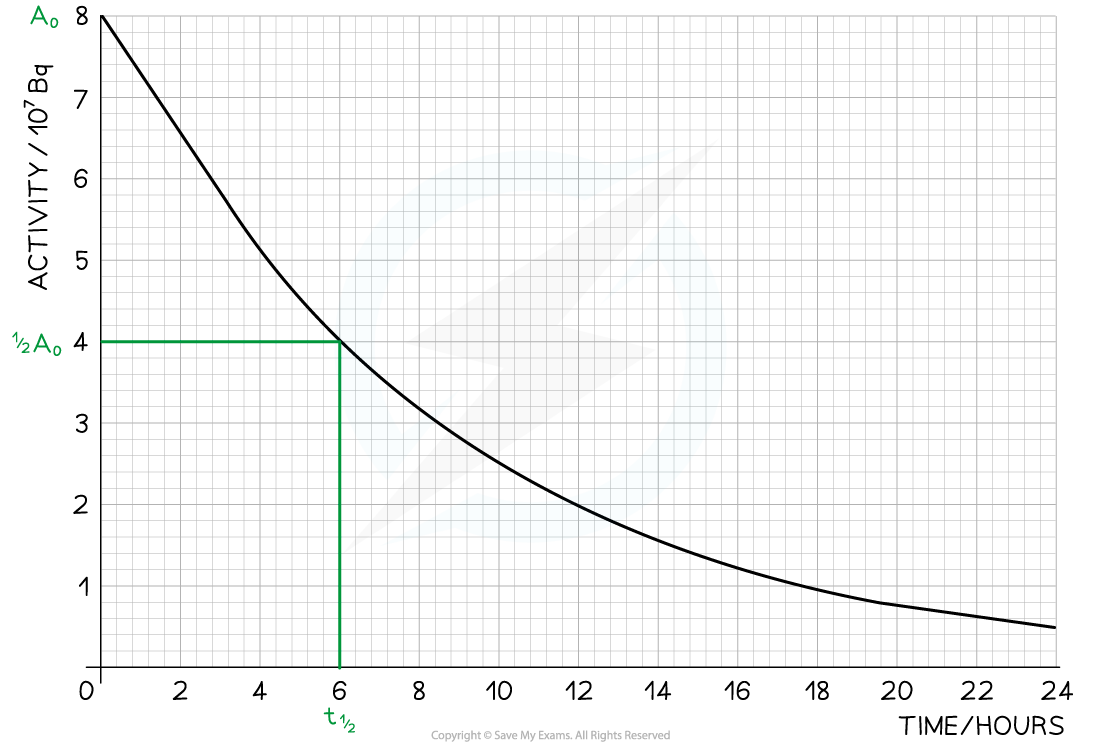
Step 2: Read the half-life from the graph
In the diagram above the initial activity, A0, is 8 × 107 Bq
The time taken to decrease to 4 × 107 Bq, or ½ A0, is 6 hours
The time taken to decrease to 2 × 107 Bq is 6 more hours
The time taken to decrease to 1 × 107 Bq is 6 more hours
Therefore, the half-life of this isotope is 6 hours
Worked Example
A particular radioactive sample contains 2 million un-decayed atoms. After a year, there is only 500 000 atoms left un-decayed.
What is the half-life of this material?
Answer:
Step 1: Calculate how many times the number of un-decayed atoms has halved
There were 2 000 000 atoms to start with
1 000 000 atoms would remain after 1 half-life
500 000 atoms would remain after 2 half-lives
Therefore, the sample has undergone 2 half-lives
Step 2: Divide the time period by the number of half-lives
The time period is a year
The number of half-lives is 2
So two half-lives is 1 year, and one half-life is 6 months
Therefore, the half-life of the sample is 6 months

Unlock more, it's free!
Was this revision note helpful?
