Biochemical Tests: Sugars & Starch (AQA A Level Biology): Revision Note
Exam code: 7402
Biochemical tests: sugars & starch
Several tests can be carried out to determine if a sample contains a certain type of sugar
The following tests are qualitative - they do not give a quantitative value as to how much of each type of molecule may be present in a sample
Sugars can be classified as reducing or non-reducing; this classification is dependent on their ability to donate electrons
Test for reducing sugars
Benedict’s reagent is a blue solution that contains copper (II) sulfate ions (CuSO4 ); in the presence of a reducing sugar, copper (I) oxide forms
Copper (I) oxide is not soluble in water, so it forms a precipitate
Benedict's test method
Add Benedict's reagent to a sample solution in a test tube
An excess of Benedict’s solution must be used so that there is more than enough copper (II) sulfate present to react with any sugar present
Heat the test tube in a water bath or a beaker of water that has been brought to a boil for a few minutes
If a reducing sugar is present, a coloured precipitate will form as copper (II) sulfate is reduced to copper (I) oxide
A positive test result is a colour change somewhere along a colour scale from blue (no reducing sugar), through green, yellow and orange (low to medium concentration of reducing sugar) to brown/brick-red (a high concentration of reducing sugar)
This test is semi-quantitative as the degree of the colour change can indicate the concentration of reducing sugar present

Test for non-reducing sugars
Non-reducing sugars are tested differently from reducing sugars because they lack the chemical groups necessary for reduction reactions
This means they cannot directly react with oxidising agents like those in Benedict's test
The addition of an acid will hydrolyse any glycosidic bonds present in any carbohydrate molecules
The resulting monosaccharides left will have an aldehyde or ketone functional group that can donate electrons to copper (II) sulfate (reducing the copper), allowing a precipitate to form
Method
Add dilute hydrochloric acid to the sample and heat in a water bath that has been brought to the boil
Neutralise the solution with sodium hydrogencarbonate
Use a suitable indicator (such as red litmus paper) to identify when the solution has been neutralised, and then add a little more sodium hydrogencarbonate as the conditions need to be slightly alkaline for Benedict’s test to work
Then carry out the Benedict’s test as normal (see above)
Test for starch
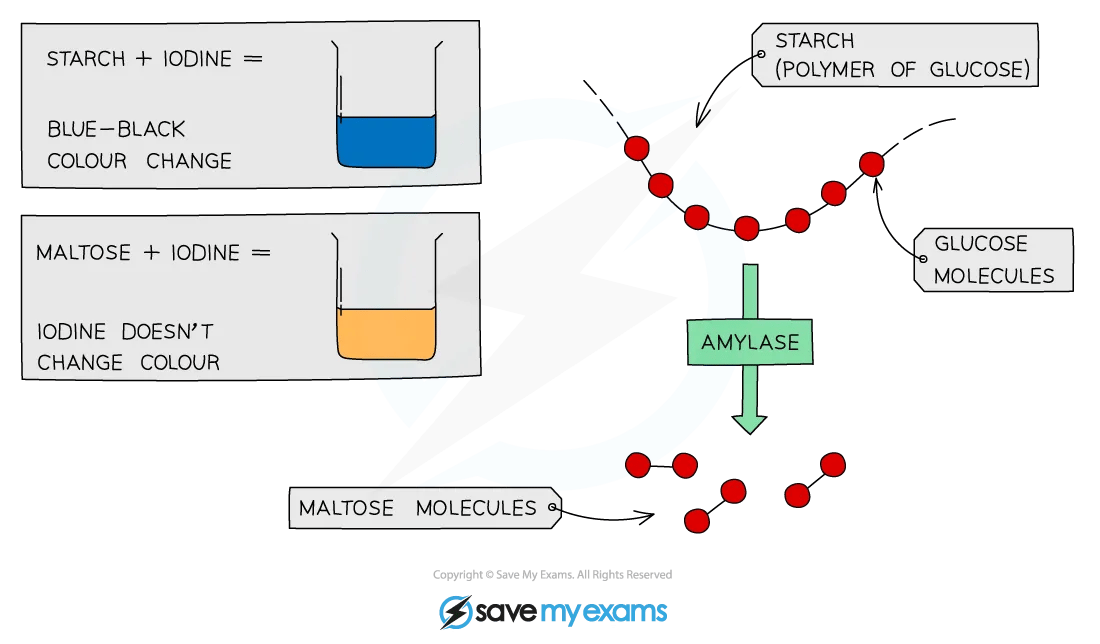
To test for the presence of starch in a sample, add a few drops of orange/brown iodine in potassium iodide solution to the sample
The iodine is in potassium iodide solution, as iodine is insoluble in water
If starch is present, iodide ions in the solution interact with the centre of starch molecules, producing a complex with a distinctive blue-black colour
This test is useful in experiments to show that starch, in a sample, has been digested by enzymes
Examiner Tips and Tricks
Be careful in your wording- do not just say 'iodine' without context that it is in solution (potassium iodide solution), as pure iodine is a very different molecule.

Unlock more, it's free!
Did this page help you?