Molar Gas Volume (DP IB Chemistry): Revision Note
Molar gas volume
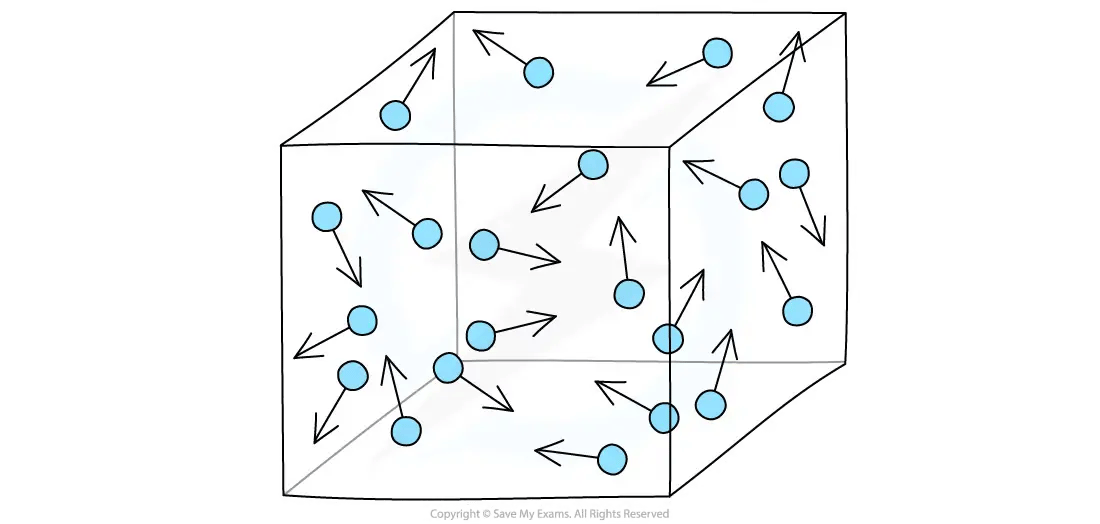
Gases in a container exert a pressure as the gas molecules are constantly colliding with the walls of the container
A particle model of a gas in a container
Changing gas volume
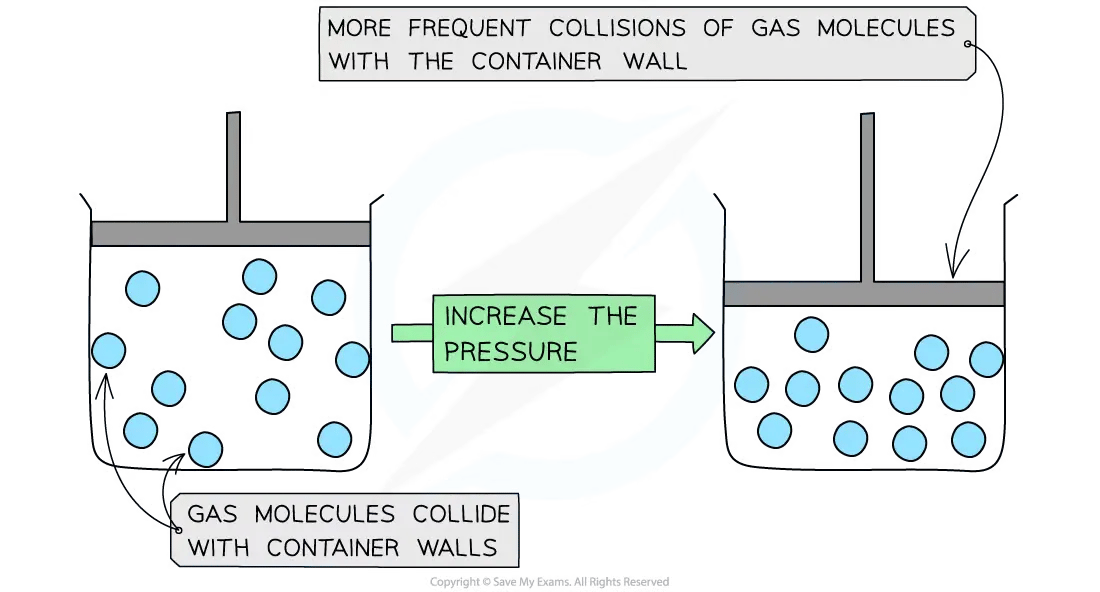
Decreasing the volume (at constant temperature) of the container causes the molecules to be squashed together which results in more frequent collisions with the container wall
The pressure of the gas increases
Gas molecule collision frequency with increasing pressure diagram
Pressure is inversely proportional to volume (when temperature is constant):
P ∝
PV = a constant
This is known as Boyle's Law
We can show a graphical representation of Boyle's Law in three different ways:
A graph of pressure of gas plotted against 1 / volume gives a straight line
A graph of pressure against volume gives a curve
A graph of PV versus P gives a straight line
Sketch graphs of Boyles' Law

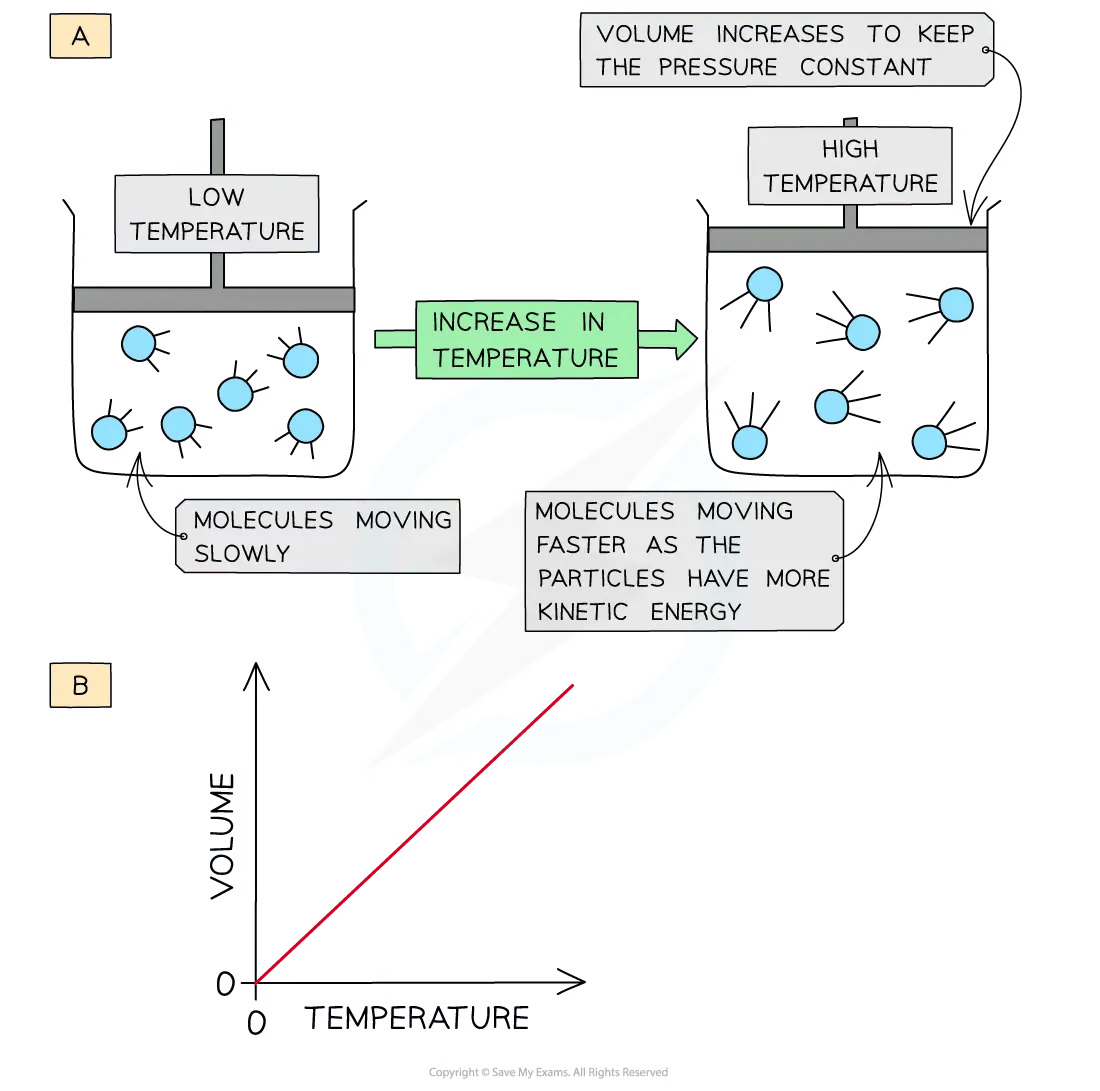
Changing gas temperature
When a gas is heated (at constant pressure) the particles gain more kinetic energy
This leads to more frequent collisions with the container walls
To keep the pressure constant, the gas must expand, so the volume increases
The volume is therefore directly proportional to the temperature in Kelvin (at constant pressure)
V ∝ T
= a constant
This is known as Charles' Law
A graph of volume against temperature in Kelvin gives a straight line
Gas molecule collision frequency with increasing temperature diagram
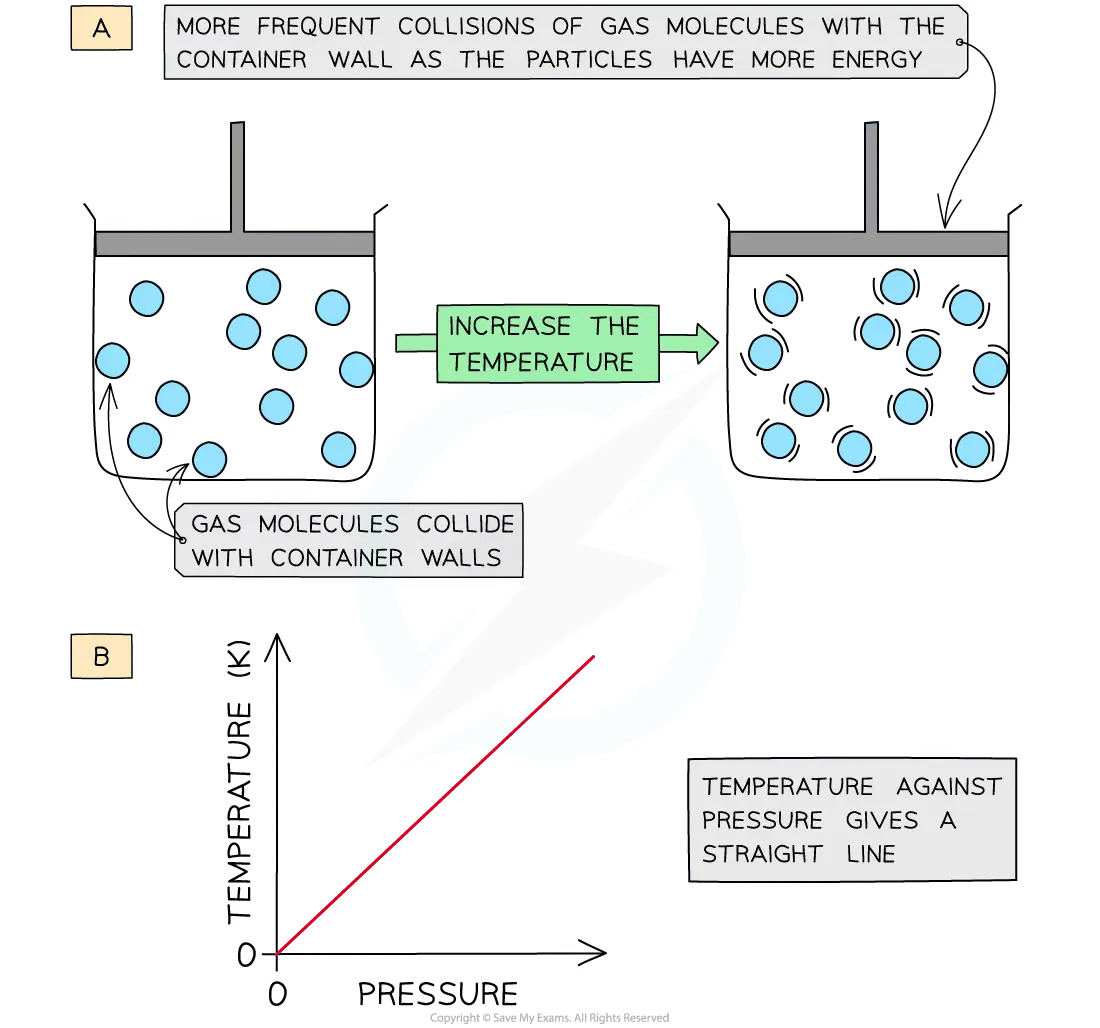
Changing gas pressure
Increasing the temperature (at constant volume) of the gas causes the molecules to gain more kinetic energy
The particles move faster and collide more frequently with the container walls
This leads to an increase in pressure
Therefore, pressure is directly proportional to temperature (in Kelvin) at constant volume
P ∝ T
= a constant
A graph of temperature in Kelvin of a gas plotted against pressure gives a straight line
Gas molecule collision frequency with increasing pressure at constant volume diagram
Pressure, volume and temperature
Combining these three relationships together:
PV = a constant
V / T = a constant
P / T = a constant
We can see how the ideal gas equation is constructed
PV / T = a constant
PV = a constant x T
This constant is made from two components, the number of moles, n, and the gas constant, R, resulting in the overall equation:
PV = nRT
Changing the conditions of a fixed amount of gas
When n (moles) and R (gas constant) are constant, the ideal gas law simplifies to:
Where
P1, V1 and T1 are the initial conditions of the gas
P2, V2 and T2 are the final conditions of the gas
The temperature must be in Kelvin
Worked Example
At 25 oC and 100 kPa a gas occupies a volume of 20 dm3. Calculate the new temperature, in oC, of the gas if the volume is decreased to 10 dm3 at constant pressure.
Answer:
Step 1: Rearrange the formula to change the conditions of a fixed amount of gas. Pressure is constant so it is left out of the formula
Step 2: Convert the temperature to Kelvin. There is no need to convert the volume to m3 because the formula is using a ratio of the two volumes
V1 = 20 dm3
V2 = 10 dm3
T1 = 25 + 273 = 298 K
Step 3: Calculate the new temperature in oC
Convert to oC = -124 oC
Worked Example
A 2.00 dm3 container of oxygen at a pressure of 80 kPa was heated from 20 oC to 70 oC The volume expanded to 2.25 dm3 . What was the final pressure of the gas?
Answer:
Step 1: Rearrange the formula to change the conditions of a fixed amount of gas
Step 2: Substitute in the values and calculate the final pressure
P1 = 80 kPa
V1 = 2.00 dm3
V2 = 2.25 dm3
T1 = 20 + 273 = 293 K
T2 = 70 + 273 = 343 K

Unlock more, it's free!
Was this revision note helpful?
