Ionisation Energy from an Emission Spectrum (HL) (DP IB Chemistry): Revision Note
Ionisation energy from an emission spectrum
Emission spectra
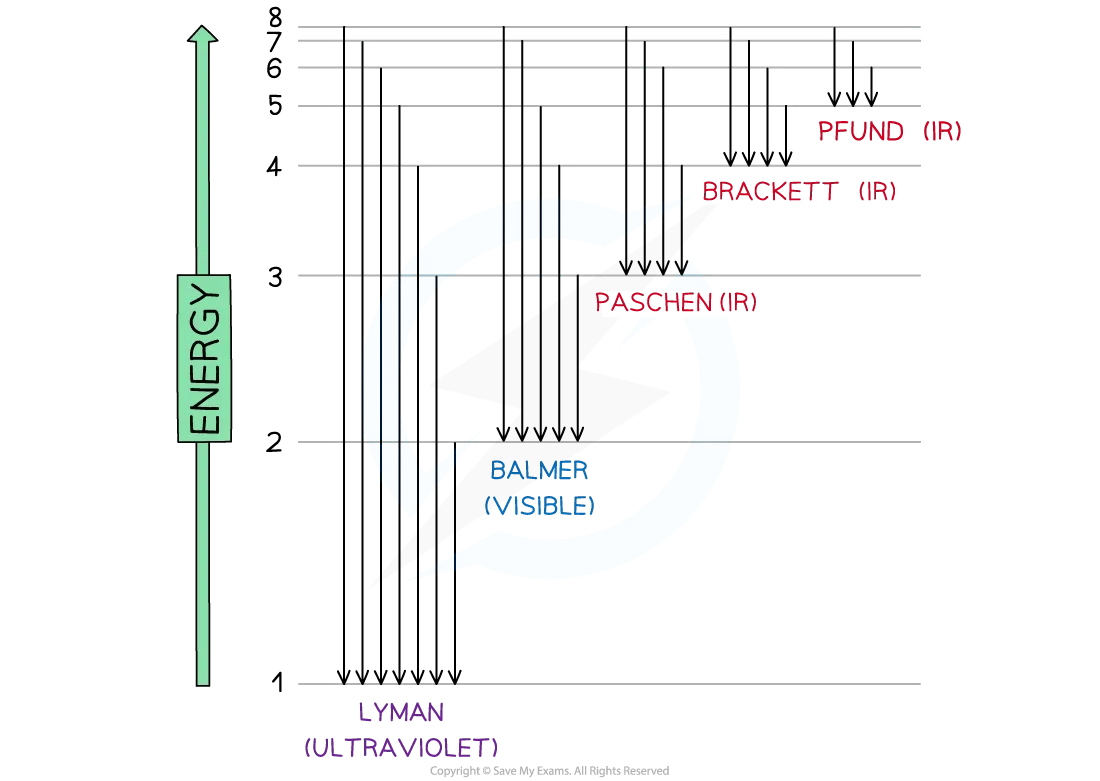
Electrons occupy energy levels (shells) around the nucleus
Energy (e.g. heat or electricity) can excite electrons to higher levels (n = 2 to ∞)
When electrons return to lower levels, they emit energy as electromagnetic radiation
A fall to n = 1 releases ultraviolet radiation:
This is the Lyman series
The amount of energy released corresponds to the difference between the energy levels
Diagram to show the release of a photon when an electron is promoted

Jumps in the hydrogen spectrum diagram
The line spectrum supports Bohr’s model, which proposes that electrons occupy discrete energy levels.
Electrons require a specific amount of energy to move between levels, like steps on a ladder.
Limitations of Bohr’s model:
Assumes electrons have fixed positions.
Assumes all energy levels are spherical.
Only accurately explains the hydrogen spectrum; fails for atoms with more than one electron.
The limit of convergence
As spectral lines are produced, they become closer together at higher energy levels
The point where lines appear to merge is called the limit of convergence
This limit represents the frequency at which the lines converge, corresponding to the energy required to remove an electron from the atom
This energy is known as the first ionisation energy
In the Lyman series (ultraviolet region), the convergence limit represents an electron falling from n = ∞ to n = 1
For hydrogen, this corresponds to a wavelength of 91.16 nm (or 91.16 × 10-9 m)
Limit of convergence diagram for hydrogen

Calculating first ionisation energy
When dealing with the Lyman series, the largest transitions represent the fall from the infinite level to n = 1
In reverse, it can be considered to be equal to the ionisation energy (note that ionisation energy is given per mole of atoms)
Therefore, the first ionisation energy (IE1) of an atom can be calculated using the frequency (or wavelength) of the convergence limit
We can do this by using the following equations
ΔE = h f
c = f λ
In order to calculate the first ionisation energy, (IE1), we must first calculate the frequency using the given data and rearranging:
c = f λ
as
f = c ÷ λ
Once we know the frequency, we can use this to calculate the ionisation energy
E = Energy (J)
h = Planck's constant (6.63 x 10–34 J s)
f = frequency (s–1)
λ = wavelength (m)
c = speed of light (3.00 x 108 m s–1)
Worked Example
The convergence limit for the sodium atom has a frequency of 1.24 × 1015 s−1. Calculate the first ionisation energy of sodium in kJ mol−1.
Answer:
Step 1: Write out the equation to calculate the first ionisation energy (IE1)
ΔE = h f
Step 2: Substitute in numbers from question and data booklet to give energy change per atom
IE1 = 6.63 × 10−34 × 1.24 × 1015
IE1 = 8.22 × 10−19 J atom−1
Step 3: Calculate the first ionisation energy per mole by multiplying by Avogadro's constant
IE1 = 8.22 × 10−19 × 6.02 × 1023
IE1 = 494 916 J mol−1
Step 4: Convert J mol−1 to kJ mol−1 by dividing by 1000
IE1 = 495 kJ mol−1
So the first ionisation energy (IE1) of sodium has been calculated as 495 kJ mol−1
Worked Example
The convergence limit for the hydrogen atom has a wavelength of 91.16 nm. Calculate the ionisation energy for hydrogen in kJ mol−1.
Answer:
Step 1: Calculate the frequency of the convergence limit, converting wavelength into m (nm to m = × 10−9)
c = f λ
f = c ÷ λ
f = 3.00 × 108 ÷ 91.16 × 10−9
f = 3.29 × 1015 s−1
Step 2: Substitute into the equation to calculate IE1 for one atom of hydrogen in J mol−1
ΔE = h f
IE1 = 6.63 × 10−34 × 3.29 × 1015
IE1 = 2.18 × 10-18 J atom−1
Step 3: Calculate IE1 for 1 mole of hydrogen atoms
IE1 = 2.18 × 10−18 × 6.02 × 1023
IE1 = 1 313 491 J mol−1
Step 4: Convert J mol−1 to kJ mol−1
IE1 = 1313 kJ mol−1
So the first ionisation energy (IE1) of hydrogen has been calculated as 1313 kJ mol−1
Examiner Tips and Tricks
These equations are found in the data booklet so you don't need to learn them
Also, be careful to calculate the first ionisation energy (IE1) per mole by using Avogadro's constant (NA) 6.02 × 1023 and converting units to kJ mol−1
Finally, when working through calculations, keep the numbers in your calculator to avoid rounding up too early.

Unlock more, it's free!
Was this revision note helpful?
