Acid-base Titrations (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Acid-base titration
Titrations are a analytical method of analysing the concentration of solutions
Titrations can determine exactly how much alkali is needed to neutralise a quantity of acid – and vice versa
You may be asked to calculate:
The moles present in a given amount
The concentration or volume required to neutralise an acid or a base
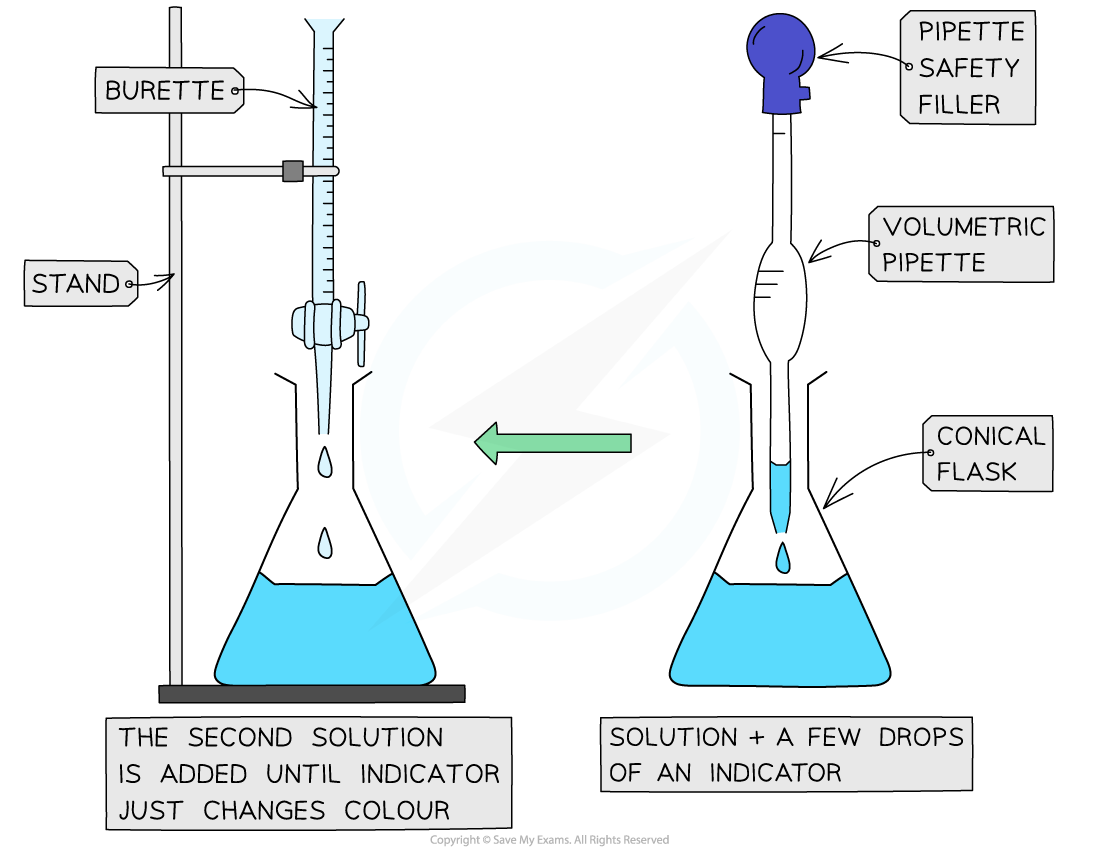
A few drops of a suitable indicator are added to show the end-point:
The end-point in a titration is when the indicator changes colour showing that the acid and base have completely reacted with each other
Specific apparatus must be used both when preparing the standard solution and when completing the titration, to ensure that volumes are measured precisely
Required apparatus
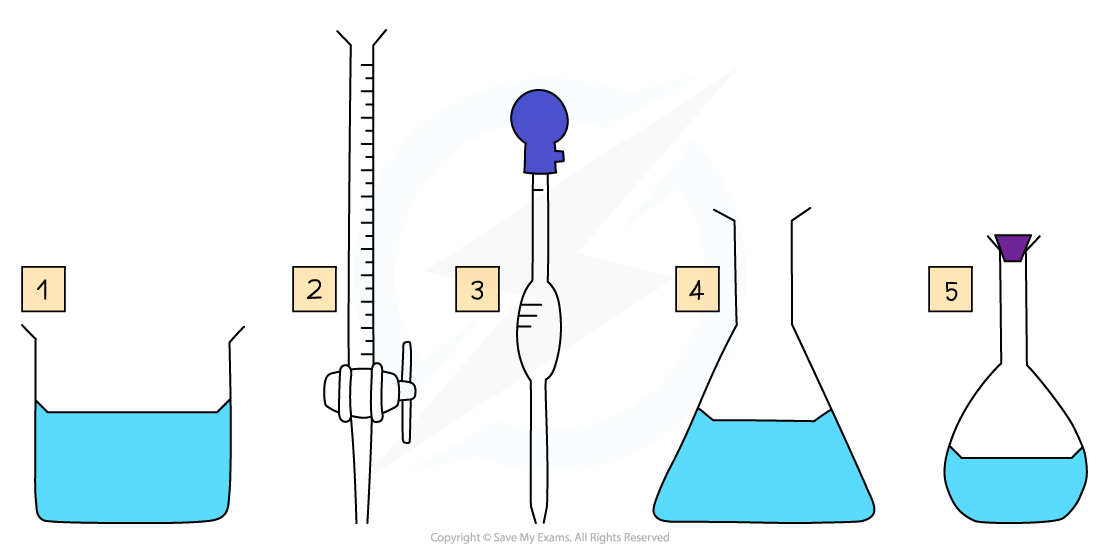
Beaker
Burette
Volumetric pipette
Conical flask
Volumetric flask
The apparatus set up
Acid-base titration calculations
Once a titration is completed and the average titre has been calculated, you can calculate the unknown variable using the formula triangle as shown below:
Formula triangle for acid-base titration calculations
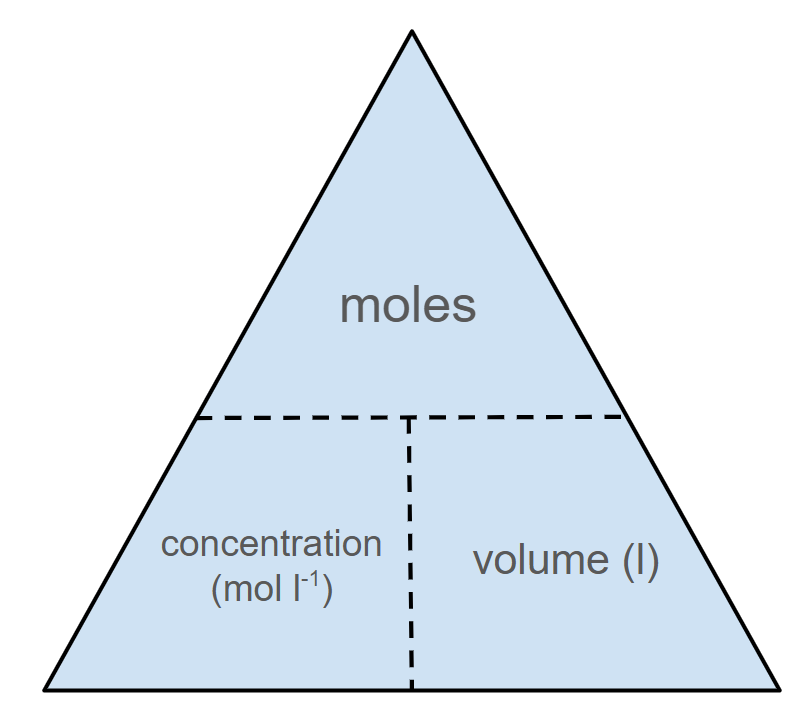
Once a titration is completed and the average titre is known, you can calculate the unknown concentration or volume
There are two ways to do this:
The "step-by-step" method
The "SQA formula" method
The step-by-step method
This method uses the mole triangle and the balanced chemical equation to work through the problem logically
It always follows three steps:
Calculate the moles of the 'known' solution:
Use the solution for which you know both the concentration and the volume
Use the formula n = c × v
Deduce the moles of the 'unknown' solution, using the mole ratio:
Look at the balancing numbers in the balanced chemical equation
Use the ratio to work out the number of moles of the 'unknown' solution that must have reacted
Calculate the unknown concentration or volume:
Now that you know the moles of the 'unknown' solution, you can use a rearranged formula to find what you're looking for:
c = n / v (to find concentration)
v = n / c (to find volume)
Worked Example
A solution of 25.0 cm3 of hydrochloric acid was titrated against a solution of 0.100 mol l-1 NaOH.
12.1 cm3 of NaOH was required for a complete reaction.
Determine the concentration of the acid.
[3]
Answer:
The balanced chemical equation is:
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Calculate the number of moles of the 'known' solution, NaOH:
moles = x concentration
moles of NaOH = 0.012 l x 0.100 mol l-1
moles of NaOH = 1.21 x 10–3 mol [1 mark]
Deduce the number of moles of the 'unknown' solution, HCl:
The acid reacts in a 1:1 ratio with the alkali
Therefore, the number of moles of HCl is also 1.21 x 10–3 mol
This is present in 25.0 cm3 of the solution (25.0 cm3 = 0.025 l) [1 mark]
Calculate the unknown concentration:
concentration =
concentration of HCl =
concentration of HCl = 0.0484 mol l-1 [1 mark]
The SQA formula method
The SQA Data Booklet provides a single formula that combines all three of the steps above into one equation
This is often a faster method to use in an exam
=
Values in the equation:
c = concentration (in mol l-1)
v = volume (in litres, l)
n = number of moles from the balanced equation (the balancing number)
'1' and '2' just stand for the two different solutions (e.g., acid and base)
How to use the SQA formula method
Write the balanced chemical equation for the reaction
This step may not be necessary if you are given the chemical equation
List all six variables (c1, v1, n1, c2, v2, n2), identifying the one you need to find
Convert all volumes to litres (l) by dividing by 1000.
Substitute the known values into the formula
Rearrange the formula for the unknown value
Solve the formula for the unknown value
Examiner Tips and Tricks
A common mistake is forgetting to convert volumes
Whichever method you use, the volume (v) must be in litres (l) for the calculation
You must divide any volumes given in cm3 by 1000
Worked Example
Calculating concentration
25.00 cm3 of 0.15 mol l-1 barium hydroxide, Ba(OH)2, was required to neutralise 12.80 cm3 of nitric acid, HNO3 , during a titration.
Calculate the concentration of HNO3 that was used. Give your answer to 2 decimal places.
Ba(OH)2 (aq) + 2HNO3 (aq) → Ba(NO3)2 (aq) + 2H2O (l)
[3]
Answer:
List all variables:
c1 (HNO3) = ?
v1 (HNO3) = 12.80 cm3 = 0.0128 l
n1 (HNO3) = 2 (from 2HNO3)
c2 (Ba(OH)2) = 0.15 mol l-1
v2 (Ba(OH)2) = 25.00 cm3 = 0.025 l
n2 (Ba(OH)2) = 1 (from 1Ba(OH)2)
[1 mark]
Substitute the values into the equation:
=
=
This simplifies to:
= 0.15 x 0.025
Rearrange the equation:
c1 (HNO3) =
[1 mark]
Solve the equation:
c1 (HNO3) = 0.59 mol l-1 to 2 dp
[1 mark]
Worked Example
Calculating volume
Calculate the volume of 0.50 mol l-1 nitric acid, HNO3, required to neutralise 25.00 cm3 of 0.80 mol l-1 potassium hydroxide, KOH. Give your answer in cm3.
KOH (aq) + HNO3 (aq) → KNO3 (aq) + H2O (l)
[3]
Answer:
List all variables:
c1 (HNO3) = 0.50 mol l-1
v1 (HNO3) = ?
n1 (HNO3) = 1 (from HNO3)
c2 (KOH) = 0.80 mol l-1
v2 (KOH) = 25.00 cm3 = 0.025 l
n2 (KOH) = 1 (from KOH)
[1 mark]
Substitute the values into the equation:
=
=
This simplifies to:
0.50 x v1 = 0.80 x 0.025
Rearrange the equation:
v1 (HNO3) =
[1 mark]
Solve the equation:
v1 (HNO3) = 0.040 l
v1 (HNO3) in cm3 = 0.040 x 1000 = 40 cm3
[1 mark]

Unlock more, it's free!
Was this revision note helpful?
