Covalent Bonding (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Covalent bonds
Non-metal atoms can share electrons with other non-metal atoms to obtain a full outer shell of electrons
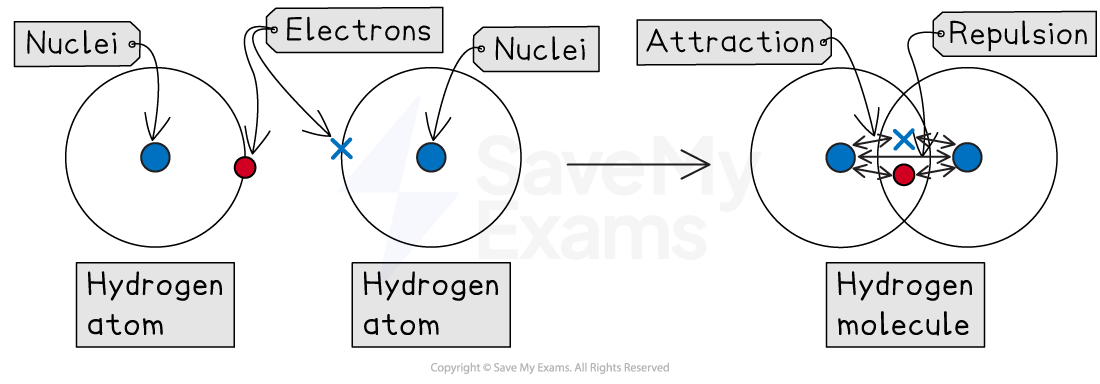
A covalent bond forms when two positive nuclei are held together by their common attraction for a shared pair of electrons
Covalent bonds between atoms are very strong
When two or more atoms are covalently bonded together, they form molecules
Formation of a covalent bond
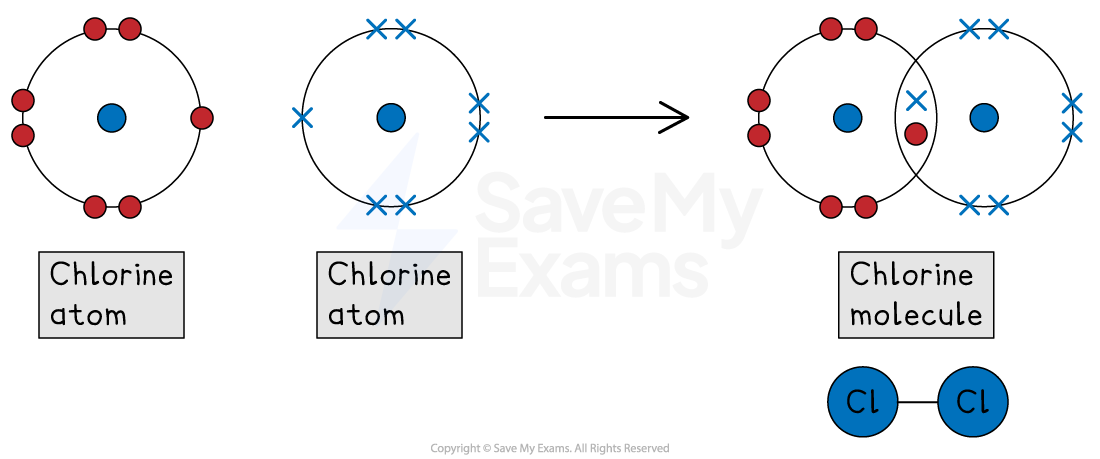
Single bonds
A single bond is formed when two atoms share one pair of electrons (a total of two electrons)
For example, chlorine (Cl2)
A chlorine atom is in Group 7, so it has 7 outer electrons
To get a full outer shell of 8, it needs to gain 1 electron
When two chlorine atoms bond, each atom shares one of its electrons with the other
This creates one shared pair in the middle
By sharing one pair, each chlorine atom can now 'count' both shared electrons as its own
This means each atom has a full outer shell of 8 (2 shared electrons + 6 unshared electrons)
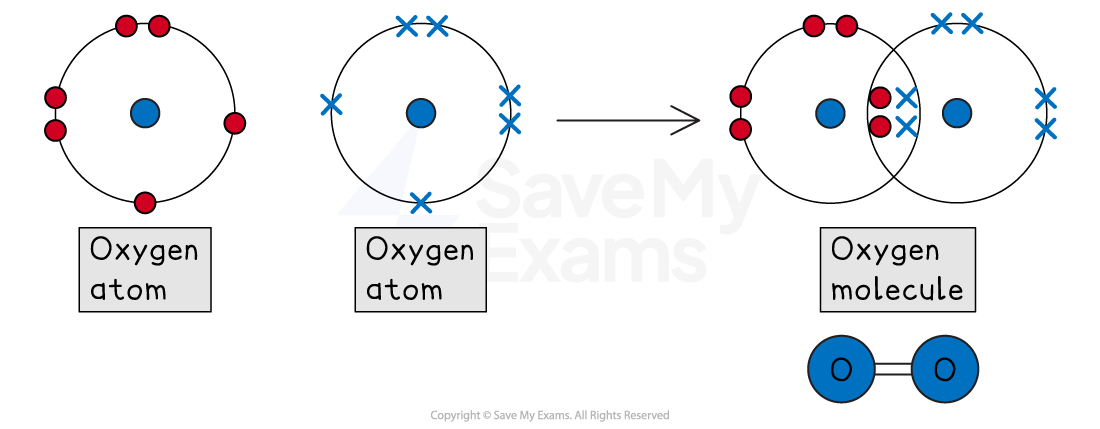
Double bonds
A double bond is formed when two atoms share two pairs of electrons (a total of four electrons).
For example, oxygen (O2)
An oxygen atom is in Group 6, so it has 6 outer electrons
To get a full outer shell of 8, it needs to gain 2 electrons
When two oxygen atoms bond, each atom shares two of its electrons with the other
This creates two shared pairs in the middle
By sharing two pairs, each oxygen atom can now 'count' all four shared electrons as its own
This means each atom has a full outer shell of 8 (4 shared electrons + 4 unshared electrons)
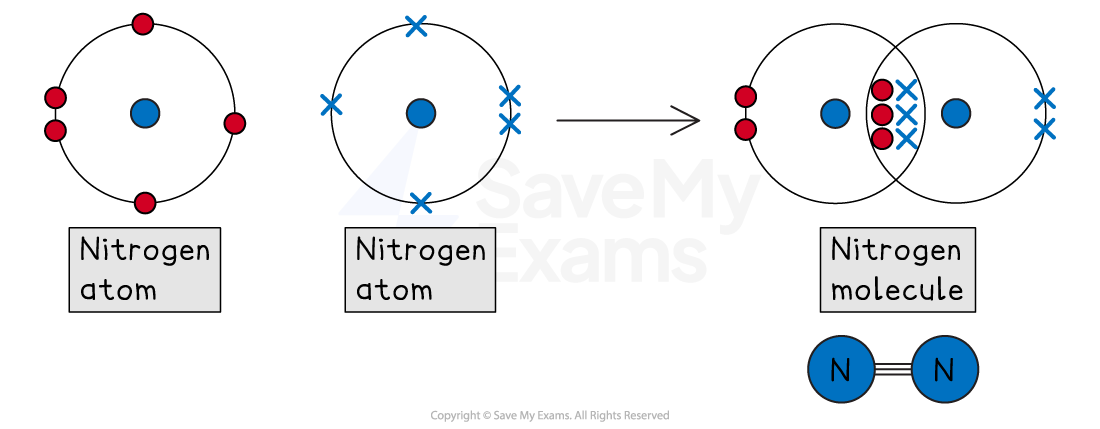
Triple bonds
A triple bond is formed when two atoms share three pairs of electrons (a total of six electrons).
For example, nitrogen (N2)
A nitrogen atom is in Group 5, so it has 5 outer electrons
To get a full outer shell of 8, it needs to gain 3 electrons
When two nitrogen atoms bond, each atom shares three of its electrons with the other
This creates three shared pairs in the middle
By sharing three pairs, each nitrogen atom can now 'count' all six shared electrons as its own
This means each atom has a full outer shell of 8 (6 shared electrons + 2 unshared electrons)
When 2 nitrogen atoms combine they form a molecule and share three pairs of electrons. This is a triple bond.
Covalent bonding diagrams
A key skill is being able to draw dot-and-cross diagrams to show how the outer electrons are shared in simple molecules
Examiner Tips and Tricks
Outer shells only:
When drawing covalent bonding diagrams, you only ever need to show the electrons in the outer shell
Do not draw the inner shells or their electrons
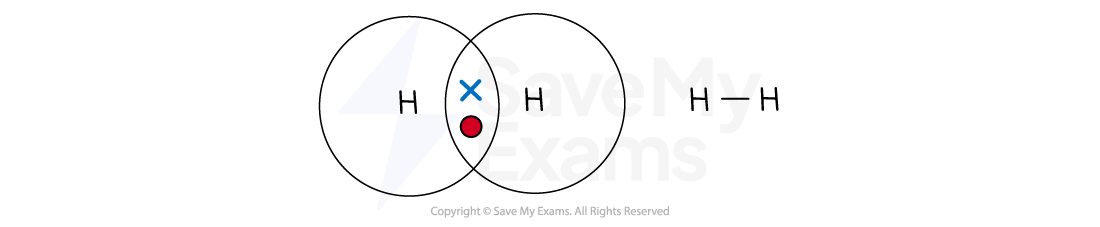
Hydrogen (H2)
A hydrogen atom has one outer electron and needs one more to get a full shell of two
The two hydrogen atoms each share their single electron, forming one shared pair
This is a single covalent bond
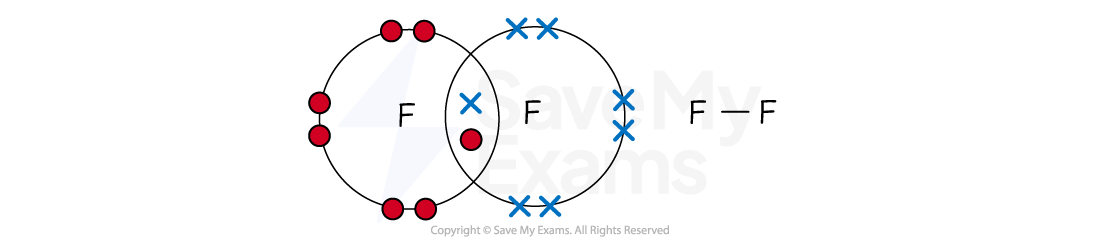
Fluorine (F2)
A fluorine atom (Group 7) has seven outer electrons and needs one more for a full shell of eight
The two fluorine atoms each share one electron, forming one shared pair
This is a single covalent bond
All the other elements of Group 7 do exactly the same because they also have 7 electrons in their outer shell
Group 7 element | Formula of molecule |
|---|---|
fluorine | F2 |
chlorine | Cl2 |
bromine | Br2 |
iodine | I2 |
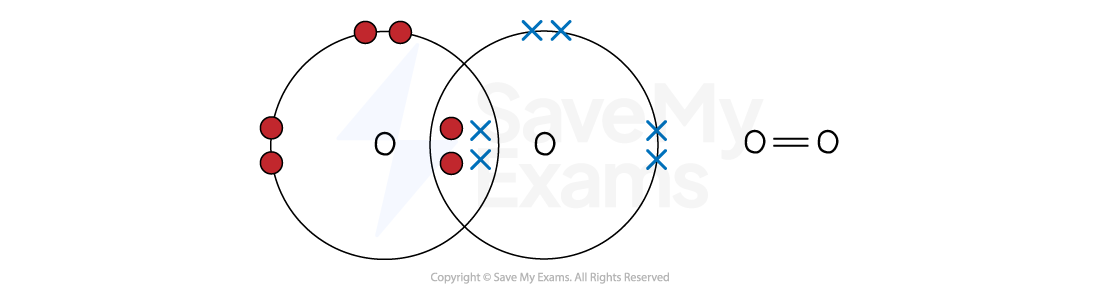
Oxygen (O2)
An oxygen atom (Group 6) has six outer electrons and needs two more for a full shell of eight
The two oxygen atoms each share two electrons, forming two shared pairs
This is a double covalent bond
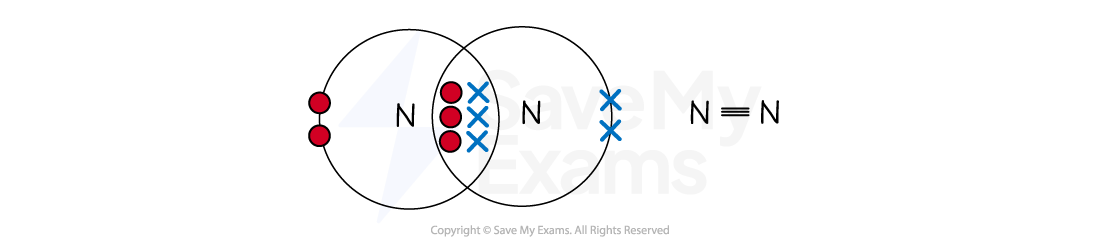
Nitrogen (N2)
A nitrogen atom (Group 5) has five outer electrons and needs three more for a full shell of eight
The two nitrogen atoms each share three electrons, forming three shared pairs
This is a triple covalent bond
Examiner Tips and Tricks
You need to remember the seven elements that exist as diatomic molecules (molecules that contain only two atoms)
Hydrogen is the simplest
The remaining six form the shape of the number 7 with its corner at the top of Group 7

The seven diatomic molecules are: hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), chlorine (Cl2), bromine (Br2) and iodine (I2)

Unlock more, it's free!
Was this revision note helpful?
