Ionic Compounds (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Forming ions
Atoms are most stable when they have a full outer shell of electrons, like the Noble Gases in Group 0 (8)
To achieve this stable electron arrangement, atoms will either lose or gain electrons
When an atom loses or gains electrons, it becomes a charged particle called an ion
Metals form positive ions
Metal atoms (from the left of the Periodic Table) have 1, 2 or 3 electrons in their outer shell
They achieve a stable electron arrangement by losing these outer electrons
When a neutral atom loses negative electrons, it is left with more positive protons.
This results in a positive ion
Example: Magnesium (Mg)
A magnesium atom has the electron arrangement 2,8,2
It loses its 2 outer electrons to achieve the stable arrangement 2,8
It now has 12 protons but only 10 electrons
So, it forms the Mg2+ ion
Non-metals form negative ions
Non-metal atoms (from the right of the Periodic Table) have 5, 6 or 7 outer electrons
They achieve a stable electron arrangement by gaining electrons to complete their outer shell
When a neutral atom gains extra negative electrons, it is left with more negatove electrons
This results in a negative ion
Example: Oxygen (O)
An oxygen atom has the electron arrangement 2,6
It gains 2 electrons to achieve the stable arrangement 2,8
It now has 8 protons but 10 electrons
So, it forms the O2- ion
Writing ion-electron equations
We can show the process of an atom losing or gaining electrons by writing an ion-electron equation
The symbol for an electron is e-
Losing electrons (metals)
When an atom loses electrons, the electrons are shown on the product (right) side of the equation
For example, magnesium:
Mg → Mg2+ + 2e-
(A magnesium atom forms a magnesium ion and two electrons)
Gaining electrons (non-metals)
When an atom gains electrons, the electrons are shown on the reactant (left) side of the equation
For example, oxygen
O + 2e- → O2-
(An oxygen atom and two electrons form an oxide ion)
Examiner Tips and Tricks
The total charge on both sides of an ion-electron equation must be balanced
For Mg → Mg2+ + 2e-:
The charge on the reactant (left) side is 0
The charge on the product (right) side is (+2) + (-2) = 0
For O + 2e- → O2-:
The charge on the reactant (left) side is 0 + (-2) = -2
The charge on the product (right) side is -2
Worked Example
An atom of aluminium has the electron arrangement 2,8,3.
a) State the charge on the ion formed by an aluminium atom.
[1]
b) Write the ion-electron equation for the formation of this ion
[1]
Answer:
a) Charge on the ion:
Aluminium is a metal, so it loses electrons.
When a neutral atom loses 3 negative electrons, it is left with 13 protons (+) and 10 electrons (-).
The resulting charge is 3+ [1 mark]
b) Ion-electron equation:
The reactant is the neutral aluminium atom, Al
The product is the ion that is formed, Al3+
Because the atom lost 3 electrons, the electrons (3e-) are shown on the product (right) side of the equation
Al → Al3++ 3e-
[1 mark]
Worked Example
An atom of fluorine has the electron arrangement 2,7.
a) State the charge on the ion formed by a fluorine atom.
[1]
b) Write the ion-electron equation for the formation of this ion.
[1]
Answer:
a) Charge on the ion:
Fluorine is a non-metal, so it gains electrons
When a neutral atom gains 1 negative electron, it has 9 protons (+) and 10 electrons (-)
The resulting charge is 1- [1 mark]
b) Ion-electron equation:
The reactant is the neutral fluorine atom, F
The product is the ion that is formed, F-
To form the ion, the atom must gain 1 electron
The electron (e-) is therefore a reactant and is written on the left side of the equation
F + e- → F-
[1 mark]
Ionic bonding & lattices
Ionic bonding
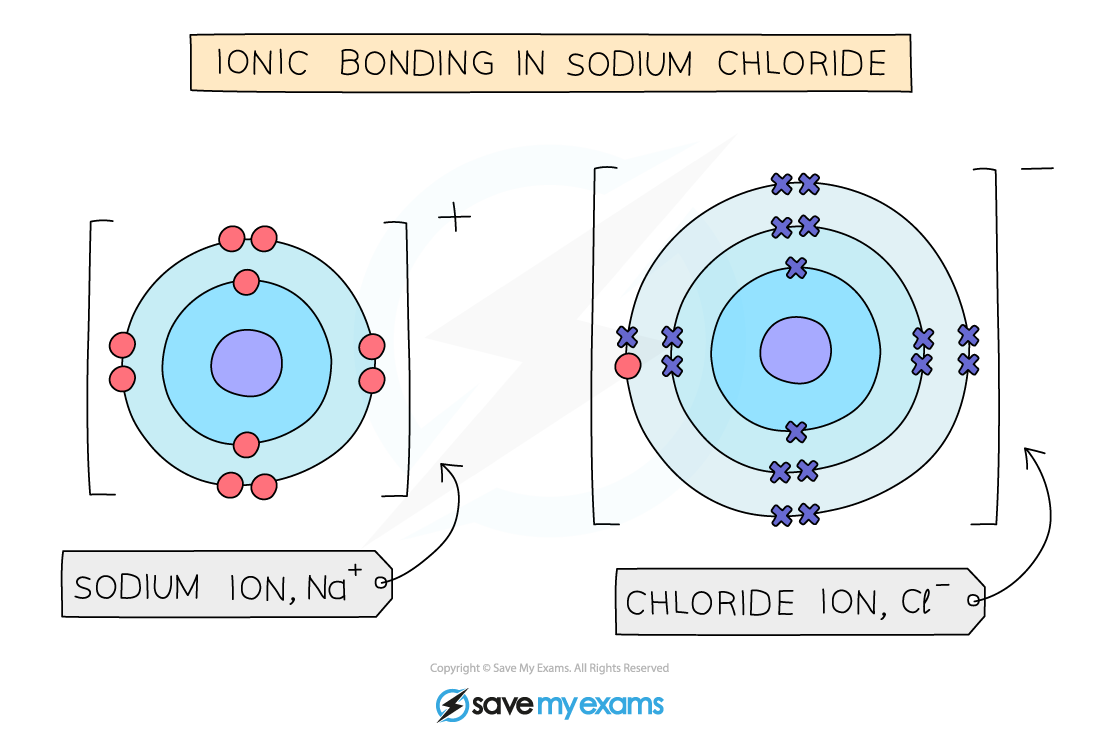
Ionic bonding occurs between metal and non-metal atoms
It involves the transfer of electrons from the metal atom to the non-metal atom
The metal atom loses its outer electrons to form a positive ion
The non-metal atom gains these electrons to form a negative ion
Ionic lattices
Ionic bonds are very strong and act in all directions
This means that a single positive ion doesn't just attract one negative ion; it attracts all the oppositely charged ions around it
As a result, ionic compounds do not form small, separate molecules
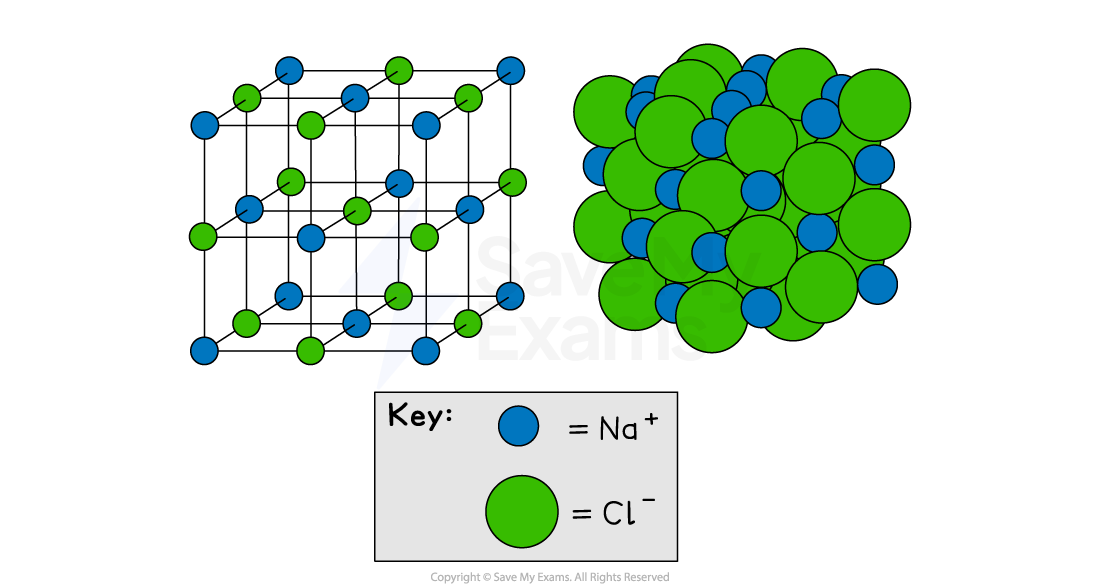
Instead, they build up into a giant, repeating, three-dimensional (3D) pattern called an ionic lattice
An ionic lattice is a regular, crystal structure
Each positive ion is surrounded by negative ions
Each negative ion is surrounded by positive ions
This arrangement ensures the electrostatic attraction is maximised, creating a very strong and stable structure
Giant ionic lattice of sodium chloride
Examiner Tips and Tricks
The chemical formula for an ionic compound, like NaCl, does not represent a single molecule. There are no "NaCl molecules"
The formula simply shows the simplest whole-number ratio of ions present in the giant lattice structure
In sodium chloride, for every one Na+ ion, there is one Cl- ion
For magnesium chloride (MgCl2), for every one Mg2+ ion, there are two Cl- ions

Unlock more, it's free!
Was this revision note helpful?
