Periodic Table & Atoms (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Using the Periodic Table
The Periodic Table is a way of organising all the known chemical elements
You can find it on page 4 of the SQA Data Booklet
How the Periodic Table is arranged
Elements are arranged in order of increasing atomic number
The atomic number is the number of protons in the nucleus of an atom
As you move from left to right across the table, each element has one more proton than the one before it
For example:
Hydrogen has atomic number 1
Helium has atomic number 2
Lithium has atomic number 3
Finding metals and non-metals
The Periodic Table has a heavy "staircase" or "zig-zag" line that divides the elements into two main categories:
Metals
Non-metals
Metals are found to the left of the staircase line
The vast majority of elements are metals
Non-metals are found to the right of the staircase line
Hydrogen (H) is an exception
It is a non-metal, even though it is located on the far left of the table
The Periodic Table
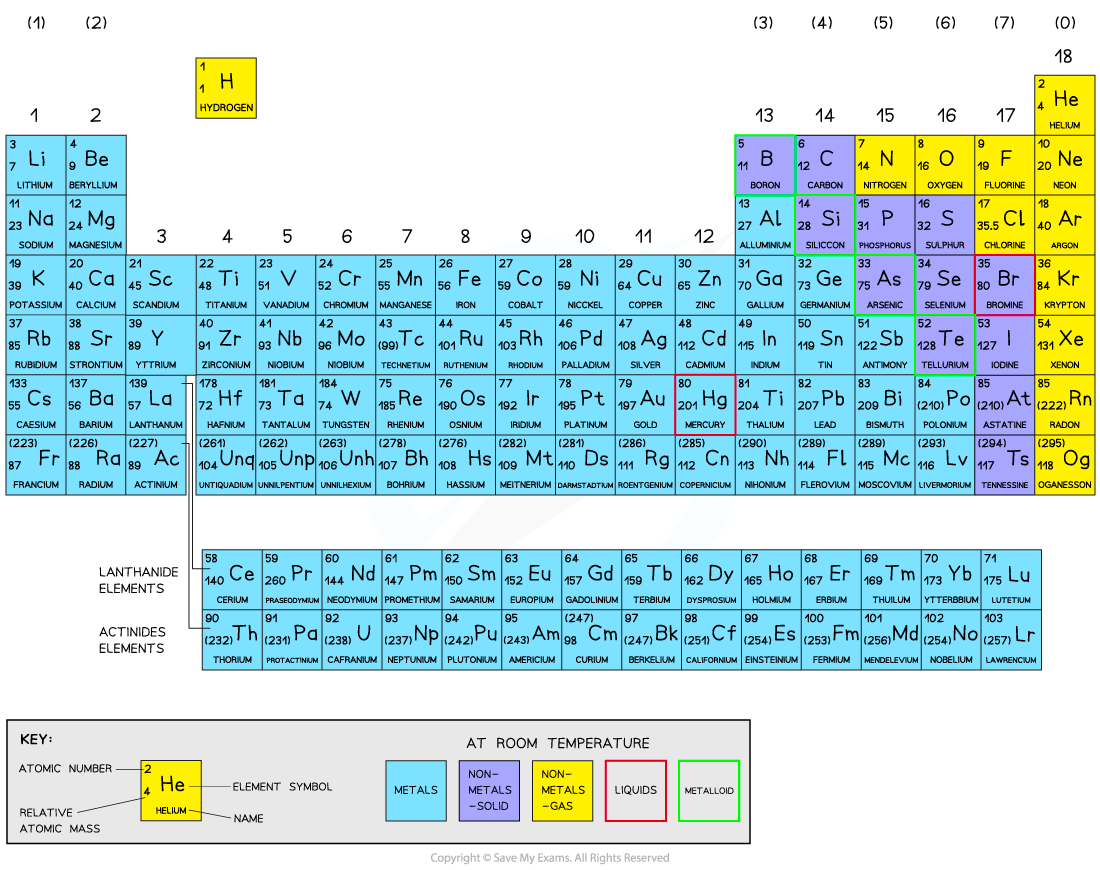
The Periodic Table is arranged in groups (columns) and periods (rows)
Examiner Tips and Tricks
In an exam, it is a good idea to draw a 'stair line' on the Periodic Table to separate the metals and non-metals
This should start above aluminium and continue as if drawing a staircase down the Periodic Table
This can be seen in the Periodic Table above, where the metals are on the left and the non-metals are on the right
Groups of the Periodic Table
Groups are the vertical columns in the Periodic Table.
All the elements within the same group share important chemical characteristics
The group number tells you the number of electrons in the outer energy level (shell) of an atom
Group number | Name of group (if applicable) | Number of outer electrons |
|---|---|---|
1 | The alkali metals | 1 |
2 | 2 | |
7 | The halogens | 7 |
0 (or 8) | The noble gases | 8 (a full outer shell)* |
*Exception: Helium is in Group 0 but has only 2 outer electrons
Why are groups important?
The number of outer electrons determines how an element reacts
All elements in a group have the same number of outer electrons
This means that they have:
Similar chemical properties:
So, they react in a very similar way
For example:
All Group 1 metals are soft, reactive metals that react vigorously with water
All Group 7 elements are reactive, coloured non-metals
The same valency:
So, they form the same number of chemical bonds
For example, elements in Group 2 have a valency of 2
This is why the chemical formulae of their compounds are often similar, such as MgCl2 and CaCl2
Electron arrangement
Electrons are not positioned randomly around the nucleus
They are found in specific energy levels called electron shells
An electron arrangement is a numerical code that shows how many electrons are in each shell of an atom
The rules for filling shells
For the first 20 elements, there are simple rules for working out the electron arrangement:
Electrons always fill the shells closest to the nucleus first
Each shell has a maximum number of electrons it can hold

A simplified model showing the electron shells
How to write an electron arrangement
There are two ways to work out an electron arrangement, using the Periodic Table (Data Booklet page 4)
Method 1: Counting from the start
Find the element's atomic number from the Periodic Table
This gives the total number of electrons
Fill the shells with electrons according to the rules above
Worked Example
State the electron arrangement of an aluminium atom.
[1]
Answer:
The atomic number of aluminium is 13
So, there are 13 electrons
The first shell holds 2 electrons, which leaves 11 electrons to place
The second shell holds 8 electrons, which leaves 3 electrons to place
The third shell holds the final three electrons
So, the electron arrangement of an aluminium atom is 2,8,3 [1 mark]
Method 2: Using the Periodic Table position
This is a faster method that shows a deeper understanding of the Periodic Table's structure
Find the Group number
This gives the number of outer electrons
Find the Period (Row) number:
This gives the total number of shells the atom has
Fill the shells:
Place the outer electrons in the outermost shell
Then, then fill the inner shells from the nucleus outwards according to the rules above
Worked Example
Give the electron arrangement of an argon atom.
[1]
Answer:
Argon is in Group 8 (0) and Period 3
Period 3 → It has 3 shells
Group 8 (0) → It has 8 electrons in its outer shell
So, the outline electron arrangement is _, _, _
Placing the outer electrons in the third shell gives _, _, 8
Then filling the inner shells
The first shell holds 2 electrons, which gives 2, _, 8
The second shell holds 8 electrons, which gives 2, 8, 8
So, the electron arrangement of an argon atom is 2,8,8 [1 mark]
Examiner Tips and Tricks
While it's important to understand these methods, remember the ultimate shortcut for the SQA National 5 course:
The Data Booklet provides the electron arrangements for many common elements on page 6
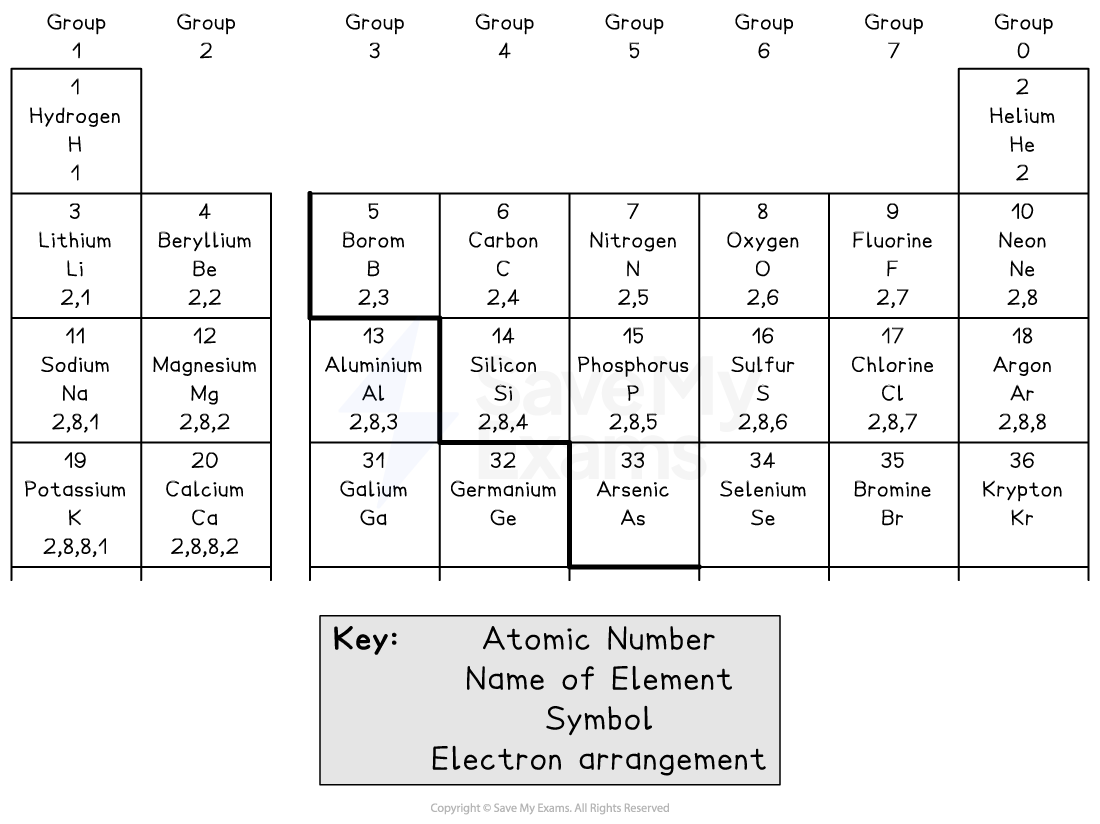
Always use this to check your answer or if you're short on time!
How to draw an electron arrangement
To draw an electron arrangement:
Show the shells as circles around the nucleus
Draw the electrons on these circles
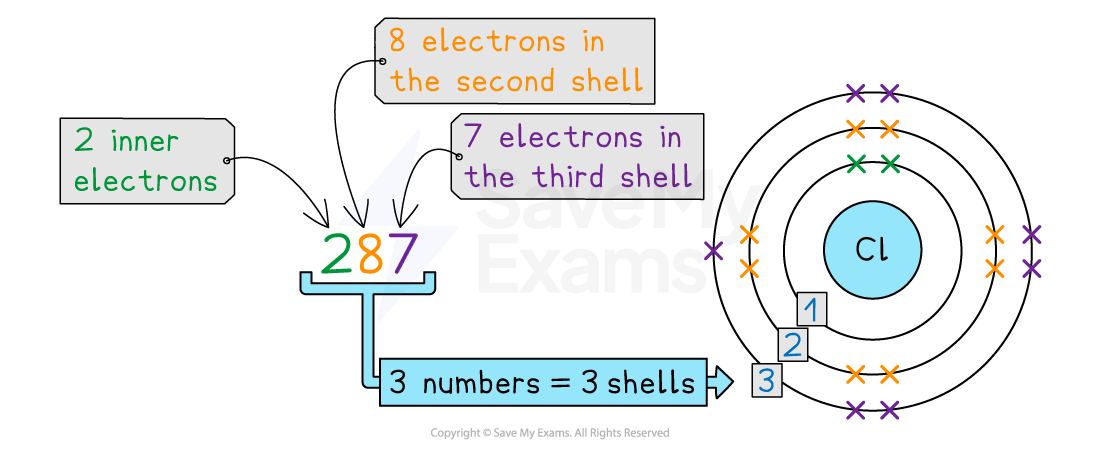
Chlorine electron arrangement
The electron arrangement of chlorine is 2,8,7
This can be deduced from the methods above or found on page 6 of the Data Booklet
This means that it has 3 electron shells
The first shell has 2 electrons
The second shell has 8 electrons
The third (outer) shell has 7 electrons
Worked Example
The diagram below represents an atom of magnesium. Complete the diagram to show the positions of all the electrons.
[1]
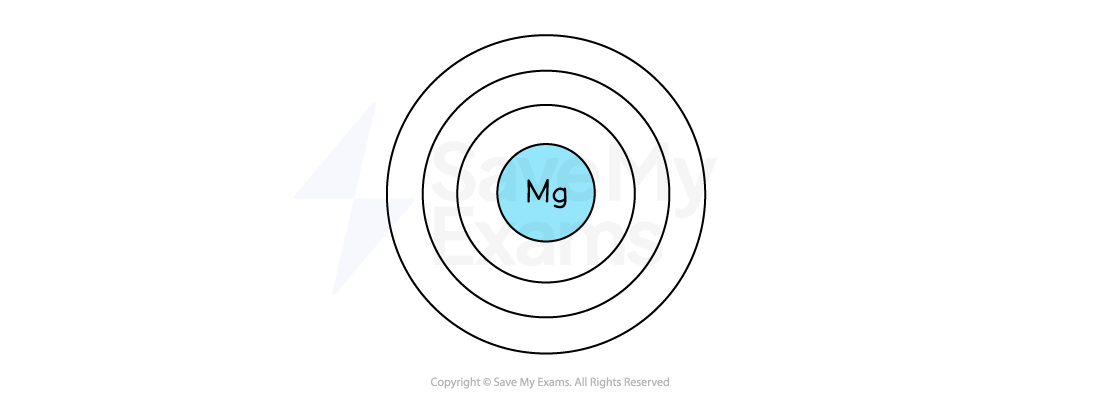
Answer:
Magnesium has 12 electrons in total
The electron arrangement of a magnesium atom is 2,8,2
The first shell has 2 electrons
The second shell has 8 electrons
The third (outer) shell has 2 electrons
So, the completed diagram for an atom of magnesium is:

[1 mark]
Worked Example
The diagram below represents an atom of phosphorus. Complete the diagram to show the positions of all the electrons.
Answer:
Phosphorus has 15 electrons in total
The electron arrangement of a phosphorus atom is 2,8,5
The first shell has 2 electrons
The second shell has 8 electrons
The third (outer) shell has 5 electrons
So, the completed diagram for an atom of phosphorus is:
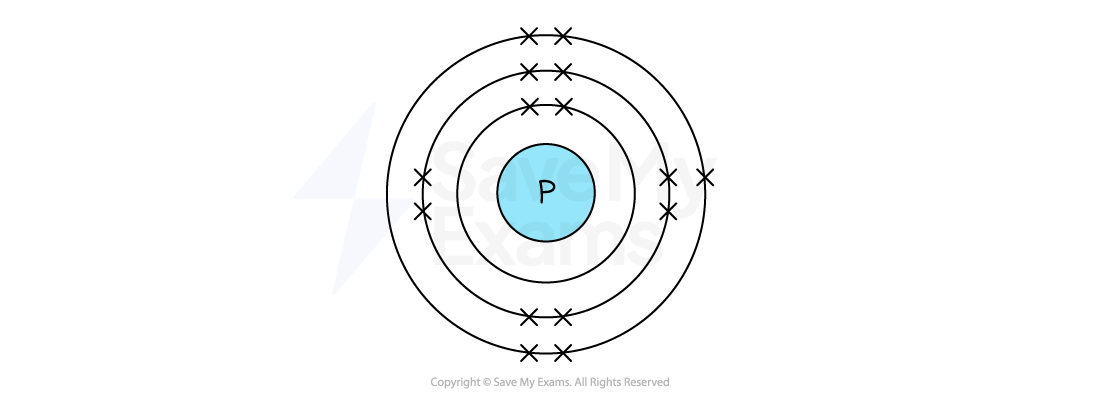
[1 mark]
Examiner Tips and Tricks
It is a good idea to draw the electrons in their shells in pairs
You will still score the marks if they aren't, as long as you have the correct number in each shell, but this makes it easier for the examiner to count

Unlock more, it's free!
Was this revision note helpful?
