Properties of Covalent Substances (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Covalent molecular substances
Covalent substances are made of non-metal atoms
They can exist in one of two structures:
Covalent molecular
Covalent network
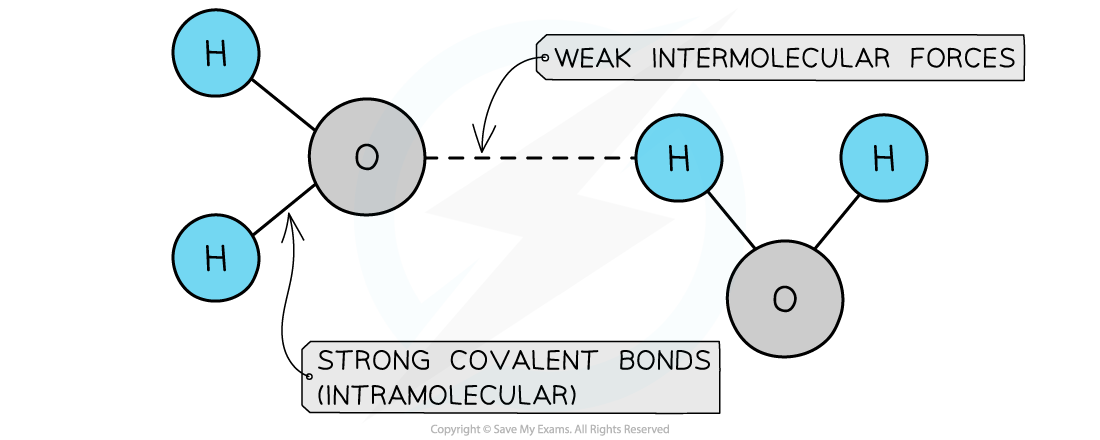
A covalent molecular structure is a small, distinct group of atoms joined by strong covalent bonds
Examples include:
Water, H2O
Carbon dioxide, CO2
Ammonia, NH3
Oxygen, O2
It is crucial to understand the two different types of forces in a covalent molecular substance:
Strong covalent bonds exist within each molecule
These hold the atoms together
Weak forces of attraction exist between the separate molecules:
These are called intermolecular forces
Properties of covalent molecular substances
Low melting and boiling points
At room temperature, covalent molecular substances are typically:
Gases
Liquids
Soft solids
This indicates they have low melting and boiling points
When a covalent molecular substance melts or boils, energy is used to overcome the weak forces of attraction between the molecules
The strong covalent bonds inside the molecules do not break
Since little energy is needed to overcome these weak forces, the melting and boiling points are low
Examiner Tips and Tricks
A common mistake is to say that covalent bonds break when a substance melts.
This is incorrect
For molecular substances, it is only the weak forces between the molecules that are overcome
Electrical conductivity
Covalent molecular substances do not conduct electricity in any state
For a substance to conduct electricity, it must contain charged particles that are free to move
Covalent molecules are neutral
Their electrons are held in fixed bonds
So, there are no free-moving charged particles available to carry a current
Covalent substances as insulators
Solubility
Most covalent molecular substances do not dissolve in water
They may, however, dissolve in other covalent solvents (such as hexane)
Covalent network structures
Some covalent substances do not form small, separate molecules
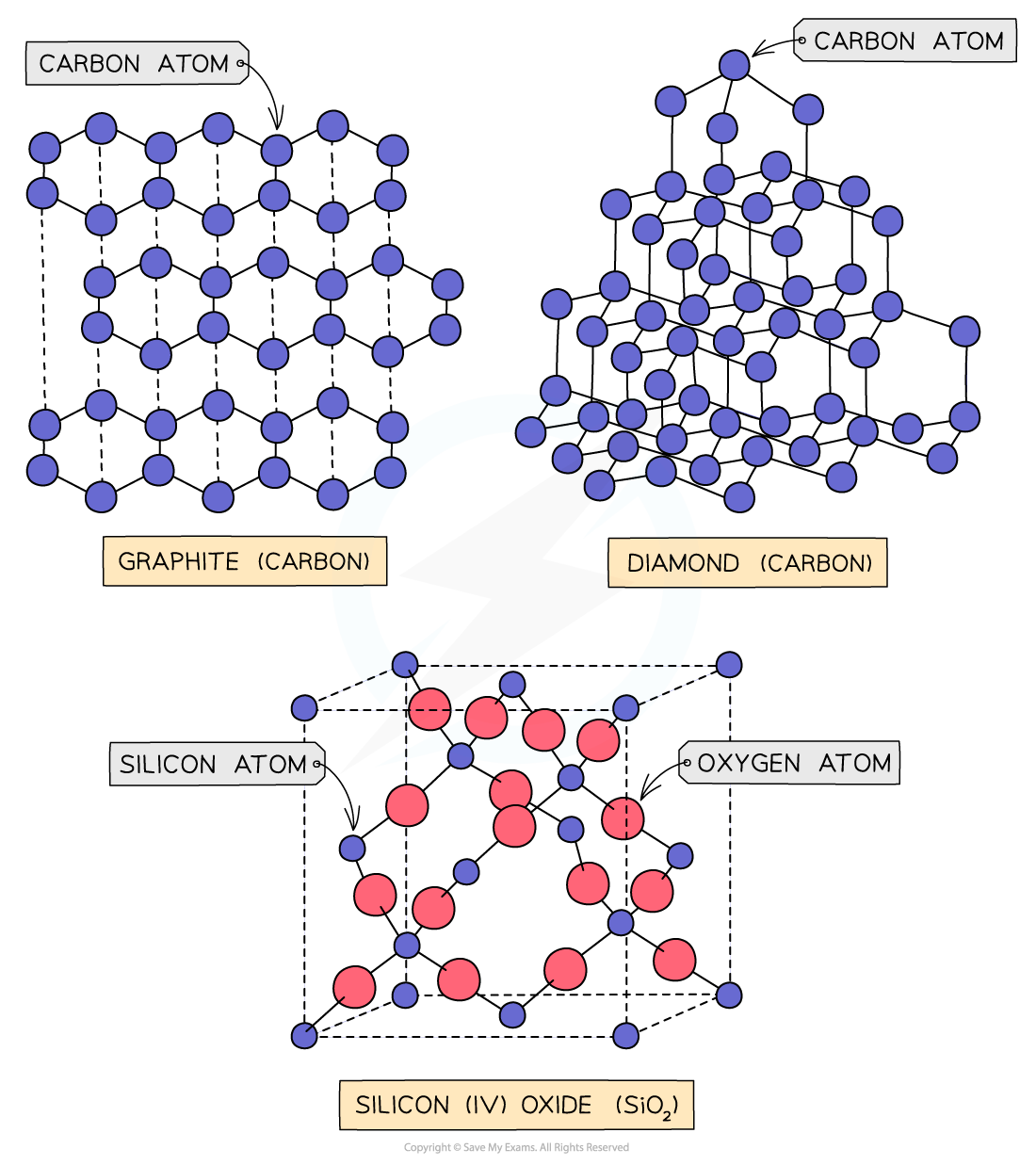
Instead, they form a covalent network structure
This is a giant three-dimensional (3D) lattice where all the atoms are held together by a vast network of strong covalent bonds
There are no weak intermolecular forces in these structures; only strong covalent bonds exist throughout.
The main examples you need to know are
Graphite
Diamond
Silicon dioxide
Graphite, diamond & silicon dioxide
Properties of covalent network structures
The properties of covalent network substances are a direct result of their very strong and rigid structure
High melting and boiling points
Covalent network structures are very hard, strong solids at room temperature.
They have extremely high melting and boiling points
To melt or boil a covalent network, a very large amount of energy is needed to break the strong covalent bonds throughout the giant structure
This is very different from molecular substances, where only weak forces are overcome
Electrical conductivity
In general, covalent network substances do not conduct electricity
In most covalent network structures, like diamond and silicon dioxide, all the outer electrons are held tightly in fixed bonds between atoms
So, there are no free-moving charged particles available to carry a current
Examiner Tips and Tricks
Graphite is the only covalent network that can conduct electricity
In graphite's layered structure, each carbon atom only bonds to three others
This leaves one delocalised electron per atom that is free to move along the layers
These mobile electrons can carry a current.
Solubility
Covalent network structures are insoluble
They do not dissolve in any solvents
The strong covalent bonds holding the atoms together in the network are too strong to be broken by the solvent particles

Unlock more, it's free!
Was this revision note helpful?
