Shapes of covalent molecules (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Shapes of simple covalent molecules
Covalent molecules are not flat; they have specific three-dimensional (3D) shapes
The shape of a simple molecule is determined by:
The number of atoms bonded to a central atom
The way the bonds are arranged in space
The pairs of electrons in the bonds repel each other and try to get as far apart in space as possible
This repulsion forces the molecule into its most stable, and therefore correct, shape
For the National 5 course, the four common shapes are:
Linear
Angular
Trigonal pyramidal
Tetrahedral
3D drawing of molecules often include wedges and dashes
These show how bonds extend in space from the central atom:
Solid wedge: The bond is coming out of the page, towards you
Dashed wedge: The bond is going into the page, away from you
Straight line: The bond lies in the plane of the page
Linear
The atoms are arranged in a straight line
There are two atoms bonded to one central atom
For example, carbon dioxide:
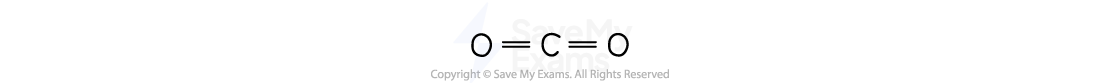
Angular
The atoms are arranged in a bent or 'V' shape
There are two atoms bonded to one central atom
For example, water:

Trigonal pyramidal
The atoms are arranged like a shallow pyramid with the central atom at the top
There are three atoms bonded to one central atom
For example, ammonia
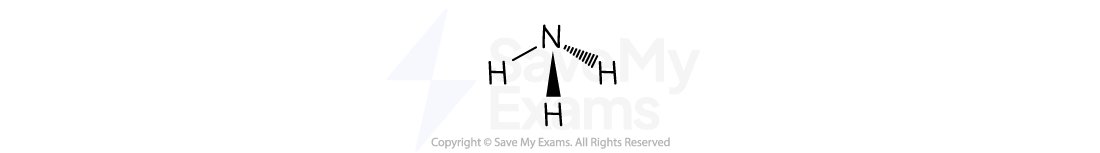
Tetrahedral
A central atom with four bonds pointing to the corners of a tetrahedron
There are four atoms bonded to the central atom
For example, methane

Examiner Tips and Tricks
Both water (angular) and carbon dioxide (linear) have a central atom bonded to two other atoms, but they have different shapes.
This is due to the presence of non-bonding pairs of electrons in water, which you don't need to explain at SQA National 5.
You just need to learn and remember the specific shapes for these common molecules.

Unlock more, it's free!
Was this revision note helpful?
