Calculations Involving the Mole (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Moles & mass
In chemistry, we need a way to measure the amount of a substance
We do this using a unit called the mole
A mole represents a specific number of particles, but for calculations, we are interested in its mass
Gram formula mass (GFM)
The gram formula mass (GFM) is the mass of one mole of a substance, measured in grams (g)
We calculate the GFM by adding up the relative atomic masses (RAM) of all the atoms in the chemical formula
You can find the relative atomic masses (RAM) for all the elements on page 7 of the SQA Data Booklet
Worked Example
What is the mass of one mole of the following compounds:
Sodium chloride, NaCl
Magnesium bromide, MgBr2
Aluminium oxide, Al2O3
Potassium nitrate, KNO3
Calcium hydroxide, Ca(OH)2
[5]
Answer
NaCl
Atoms: 1 x Na, 1 x Cl
RAMs: Na = 23, Cl = 35.5
Mass of Na = 1 x 23 = 23
Mass of Cl = 1 x 35.5 = 35.5
Total GFM: 23 + 35.5 = 58.5 [1 mark]
MgBr2
Atoms: 1 x Mg, 2 x Br
RAMs: Mg = 24.5, Br = 80
Mass of Mg = 1 x 24.5 = 24.5
Mass of Br = 2 x 80 = 160
Total GFM: 24.5 + 160 = 184.5 [1 mark]
Al2O3
Atoms: 2 x Al, 3 x O
RAMs: Al = 27, O = 16
Mass of Al = 2 x 27 = 54
Mass of O = 3 x 16 = 48
Total GFM: 54 + 48 = 102 [1 mark]
KNO3
Atoms: 1 x K, 1 x N, 3 x O
RAMs: K = 39, N = 14, O = 16
Mass of K = 1 x 39 = 39
Mass of N = 1 x 14 = 14
Mass of O = 3 x 16 = 48
Total GFM: 39 + 14 + 48 = 101 [1 mark]
Ca(OH)2
Atoms: 1 x Ca, 2 x O, 2 x H (Remember the 2 outside the bracket applies to both the O and H)
RAMs: Ca = 40, O = 16, H = 1
Mass of Ca = 1 x 40 = 40
Mass of O = 2 x 16 = 32
Mass of H = 2 x 1 = 2
Total GFM: 40 + 32 + 2 = 74 [1 mark]
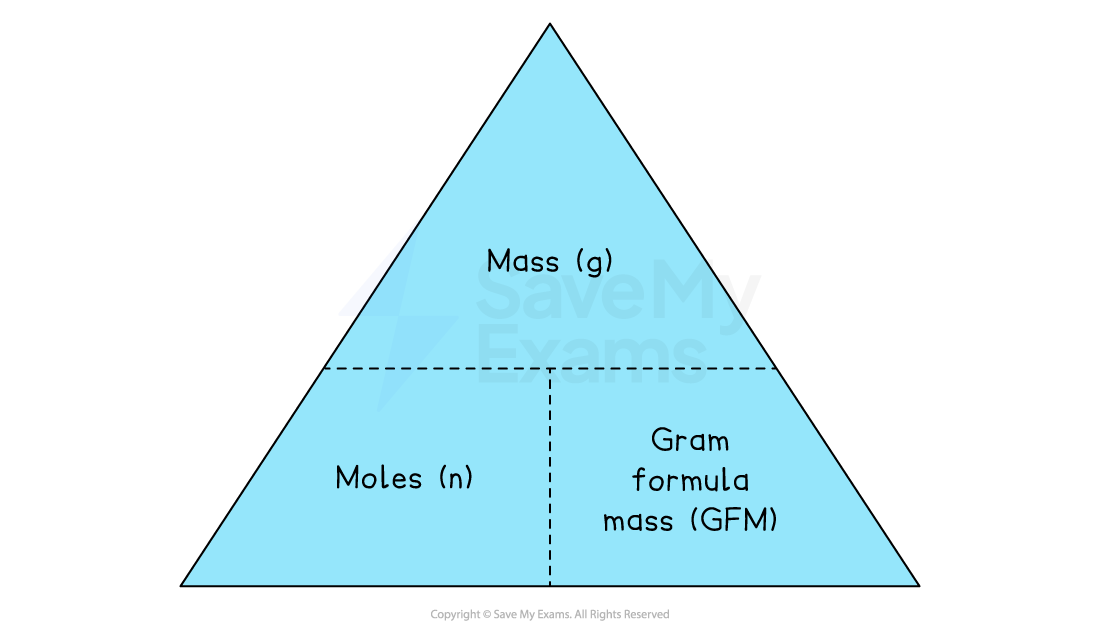
The mole triangle
The number of moles (n), the mass in grams (mass), and the Gram Formula Mass (GFM) are all related.
We can use a triangle to help us with the calculations
The mole triangle
Worked Example
Calculating moles
How many moles are in 8g of methane (CH4)?
[2]
How many moles are in 3.4 g of ammonia, NH3?
[2]
Answer 1
Step 1: Calculate GFM of CH4:
C + (4 x H)
12 + (4 x 1) = 16 [1 mark]
Step 2: Use the mole triangle to find the number of moles (n)
n =
n = = 0.5 moles [1 mark]
Answer 2
Step 1: Calculate the GFM of NH3
1 x N, 3 x H
14 + 3 = 17 [1 mark]
Step 2: Use the mole triangle to find the number of moles (n)
n =
n = = 0.2 moles [1 mark]
Worked Example
Calculating mass
What is the mass of 0.5 moles of carbon dioxide, CO2?
What is the mass of 0.2 moles of aluminium hydroxide, Al(OH)3?
Answer 1
Step 1: Calculate GFM of CO2:
C + (2 x O)
GFM = 12 + (2 x 16) = 44 [1 mark]
Step 2: Calculate the mass of CO2 using the mole triangle:
mass = n x GFM
mass = 0.5 x 44
mass = 22 g [1 mark]
Answer 2
Step 1: Calculate GFM of Al(OH)3 formula for
Al + (3 x O) + (3 x H)
GFM = 27 + 48 + 3 = 78 [1 mark]
Step 2: Calculate the mass of Al(OH)3 using the mole triangle:
mass = n × GFM
mass = 0.2 × 78
mass = 15.6 g [1 mark]
Examiner Tips and Tricks
Always show your workings in calculations as it's easier to check for errors and you may pick up credit if you get the final answer wrong.
Reacting mass calculations
A balanced chemical equation is like a recipe
It tells you the ratio of moles of reactants that are needed and products that are made
We can use this ratio to calculate the mass of a substance in a reaction if we know the mass of another
The 3-Step Method
To solve these problems, we always follow the same three steps:
Calculate moles of the 'known':
Use the mass given in the question and the GFM to find the number of moles of your starting substance.
n =
Use the mole ratio:
Look at the balancing numbers in the balanced equation to find the ratio between your 'known' substance and the substance you want to find out about (the 'unknown')
Calculate mass of the 'unknown':
Use the new number of moles and the GFM of the 'unknown' substance to calculate its mass
mass = n × GFM
Worked Example
The equation for magnesium burning is:
2Mg (s) + O2 (g) ⟶ 2MgO (s)
Calculate the mass of magnesium oxide, MgO, that can be made by completely burning 6.0 g of magnesium, Mg.
(Use RAMs from page 7 of the Data Booklet: Mg = 24.5, O = 16)
[3]
Answer:
Calculate moles of the 'known' (Mg)
RAM of Mg = 24.5
n =
n = = 0.245 moles of Mg [1 mark]
Use the mole ratio
From the balanced equation, find the ratio between Mg and MgO
2Mg : 2MgO
Simplify the ratio to 1 : 1
This means 0.245 moles of Mg will produce 0.245 moles of MgO [1 mark]
Calculate mass of the 'unknown' magnesium oxide
GFM of MgO = 24.5 + 16 = 40.5 g
mass = n × GFM
mass = 0.245 × 40.5
mass = 9.92 g of MgO [1 mark]
Worked Example
The equation for the decomposition of aluminium oxide is:
2Al2O3 ⟶ 4Al + 3O2
Calculate the maximum possible mass of aluminium, Al, in tonnes, that can be produced from 51 tonnes of aluminium oxide, Al2O3.
(Use RAMs from page 7 of the Data Booklet: Al = 27, O = 16)
[3]
Answer:
Calculate moles of the 'known', Al2O3
GFM of Al2O3 = (2 x 27) + (3 x 16) = 54 + 48 = 102
n =
n = = 500 000 moles of Al2O3 [1 mark]
Use the mole ratio
From the balanced equation, find the ratio between Al2O3 and Al.
2Al2O3 : 4Al
Simplify the ratio to 1 : 2
This means 500 000 moles of Al2O3 will produce (500 000 x 2) = 1 000 000 moles of Al [1 mark]
Calculate mass of the 'unknown', Al
RAMof Al = 27
mass = n × GFM
mass = 1 000 000 × 27
mass = 27 000 000 g = 27 tonnes of Al [1 mark]
Examiner Tips and Tricks
In multi-step calculations, a very common mistake is rounding the answer from one step before using it in the next.
This can create a "rounding error", making your final answer slightly inaccurate and potentially costing you the final mark.
Only round the final answer that you write on the answer line. If the question doesn't specify, rounding to one or two decimal places is usually fine.

Unlock more, it's free!
Was this revision note helpful?
