Chemical Formulae (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Chemical formulae and names
To write the correct chemical formula for a compound, you need to know the 'combining power' of the atoms or ions involved
This is called the valency
Valency indicates how many chemical bonds an atom can form
By learning the valency rules and other clues in a name, you can build any formula you need
Valency
For Groups 1 - 4:
valency = the group number
For Groups 5–7:
valency = (8 - the group number)
For Group 0 (8), the valency is 0
Group number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 0 (Noble gases) |
|---|---|---|---|---|---|---|---|---|
Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
Examiner Tips and Tricks
The Periodic Table is on page 4 of the Data Booklet
You can use it in the exam to find the group number and therefore the valency of each element
Roman numerals in names of compounds
The valency of an element helps determine the charge on its ion
For example, magnesium is in Group 2:
It has a valency of 2
It forms an Mg2+ ion
However, some metals, especially the transition metals in the middle of the Periodic Table, can have more than one valency
This means that they can form more than one type of ion
For example, iron can form an Fe2+ ion or an Fe3+ ion
To avoid confusion, a Roman numeral is used in the name to state the exact valency (and therefore the charge) of the metal in that specific compound:
Roman numeral | Valency | Example ion | Example compound |
|---|---|---|---|
(I) | 1 | copper(I), Cu+ | copper(I) oxide |
(II) | 2 | iron(II), Fe2+ | iron(II) chloride |
(III) | 3 | iron(III), Fe3+ | iron(III) oxide |
(IV) | 4 | lead(IV), Pb4+ | lead(IV) chloride |
Examiner Tips and Tricks
The Roman numeral tells you the valency and charge of the metal ion in that compound
It does not tell you how many atoms of that metal are in the formula
Prefixes in the names of compounds
Covalent compounds (made only of non-metals) use prefixes in their names to show exactly how many atoms of each element are in one molecule
Prefix | Number of atoms | Example compound name | Example compound formula |
|---|---|---|---|
mono– | 1 | Carbon monoxide | CO |
di– | 2 | Carbon dioxide | CO2 |
tri– | 3 | Nitrogen trihydride (ammonia) | NH3 |
tetra– | 4 | Carbon tetrachloride | CCl4 |
penta– | 5 | Phosphorus pentachloride | PCl5 |
hexa– | 6 | Sulfur hexafluoride | SF6 |
Examiner Tips and Tricks
The prefixes di–, tri–, tetra– are the most common at National 5
Prefixes tell you the exact number of atoms in one molecule
They do not tell you the valency
This prefix system is used only for covalent compounds, not ionic ones
Chemical formulae of covalent substances
Covalent molecular formulae
Covalent molecular substances are made of individual, separate molecules.
The chemical formula for a molecular substance tells you the exact number of atoms in one single molecule
Water (H2O)
The H2O formula means that one molecule of water contains exactly:
2 hydrogen atoms
1 oxygen atom
Methane (CH4)
The CH4 formula means one molecule of methane contains exactly:
1 carbon atom
4 hydrogen atoms
Examiner Tips and Tricks
Covalent molecular formulae cannot be simplified
For example, ethane (C2H6)
One molecule of ethane contains 2 carbon and 6 hydrogen atoms
So, the formula cannot be simplified to CH3, as that would not represent a real molecule of ethane
Covalent network formulae
Covalent network substances are made of a giant, continuous lattice of atoms. There are no individual molecules
The chemical formula for a covalent network substance gives the simplest whole-number ratio of atoms in the giant structure
Silicon dioxide (SiO2)
The SiO2 formula means that for every 1 silicon atom in the network, there are 2 oxygen atoms
This formula represents the simplest ratio of atoms in the giant covalent network of silicon dioxide
Diamond (C)
The formula for diamond is just C
This is because the entire covalent network is made of only carbon atoms
Summary
Feature | Covalent molecular | Covalent network |
|---|---|---|
Structure | Made of individual, separate molecules | A giant, continuous lattice of atoms |
What formula shows | The exact number of atoms in one molecule | The simplest ratio of atoms in the structure |
Example | Ammonia (NH3) | Silicon Carbide (SiC) |
Chemical formulae of ionic compounds
Many compounds contain group ions
These are ions that are made of more than one type of atom bonded together
You can find the names and formulae of selected group ions on page 8 of the SQA Data Booklet
Finding the valency of a group ion
The valency of a group ion is the number in its charge:
Ammonium (NH4+) has a charge of 1+, so its valency is 1
Nitrate (NO3-) has a charge of 1-, so its valency is 1
Sulfate (SO42-) has a charge of 2-, so its valency is 2
Phosphate (PO43-) has a charge of 3-, so its valency is 3
How to write an ionic formula
Ionic compounds typically have no overall charge
This means that the size of any positively charged ion is cancelled by the size of any negatively charged ion
Careful: This should not be confused with an atom having no overall charge
The formula of an ionic compound can be determined if you know the charge on the ions
There are two methods to do this:
Direct comparison
Swap-and-drop
The direct comparison method
This method compares the charges of the ions in the compound
Iron(II) sulfate
The iron(II) ion is Fe2+
It has a 2+ or +2 charge
The sulfate ion is SO42–
It has a 2– or –2 charge
The 2+ and 2- charges cancel each other out
This means that one Fe2+ ion is needed to cancel out the 2- charge of one SO42- ion
Therefore, the formula of iron(II) sulfate is FeSO4
The swap-and-drop method
When the ions in the ionic compound have different charges, it can be easier to use the swap-and-drop method
Careful: If you use this method with ions that have the same charge, then you must give the simplest whole number ratio to get the correct answer
Copper(II) chloride
The copper(II) ion is Cu2+
It has a 2+ or +2 charge
The chloride ion is Cl–
It has a 1– or –1 charge
The size of the charge on the copper(II) ion indicates the number of chloride ions needed
The size of the charge on the chloride ion indicates the number of copper(II) ions needed
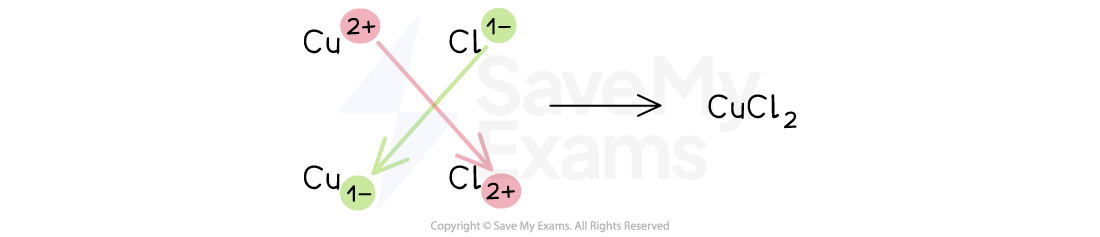
Therefore, the formula of copper(II) chloride is CuCl2
Formatting rules
You must write formulae correctly to get the marks
Subscripts: The small numbers showing how many atoms/ions there are must be written low
H2O (correct)
H2O (incorrect)
H2O (incorrect)
Superscripts: The charges on ions must be written high
Mg2+ (correct)
Mg2+ (incorrect)
Mg2+ (incorrect)
Worked Example
The compound produced in the reaction between iron wool and chlorine contains the ions Fe3+ and Cl–.
a) Give the formula of this compound.
[1]
b) State the name of this compound.
[1]
Answers:
Part a)
Using the direct comparison method:
The iron ion is Fe3+, which means that it has a 3+ or +3 charge
The chloride ion is Cl–, which means that it has a 1– or –1 charge
The charges do not cancel each other out
Mathematically, (+3) + (–1) ≠ 0
Three Cl– ions are needed to cancel the +3 charge on Fe3+
Therefore, the formula is FeCl3 [1 mark]
Using the swap-and-drop method

Therefore, the formula is FeCl3 [1 mark]
Part b)
The metal ion is Fe3+, so the name must include the Roman numeral (III)
The chlorine name will change to chloride
Therefore, the name is iron(III) chloride [1 mark]
Worked Example
What is the formula for lead(IV) oxide?
[1]
Answer:
Using the direct comparison method:
The name of the compound has IV which means the lead ion is Pb4+
The oxide ion has the charge 2-
The charges do not cancel each other out
Mathematically, (+4) + (–2) ≠ 0
Two O2- ions are required to cancel the charge of the Pb4+ ion
Therefore, the formula is PbO2 [1 mark]
Using the swap and drop method:
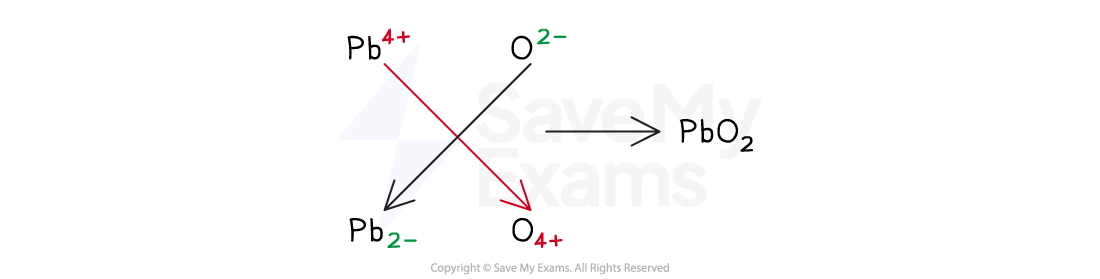
Therefore, the formula is PbO2 [1 mark]

Unlock more, it's free!
Was this revision note helpful?
