Solution Calculations (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Solutions
What is a solution?
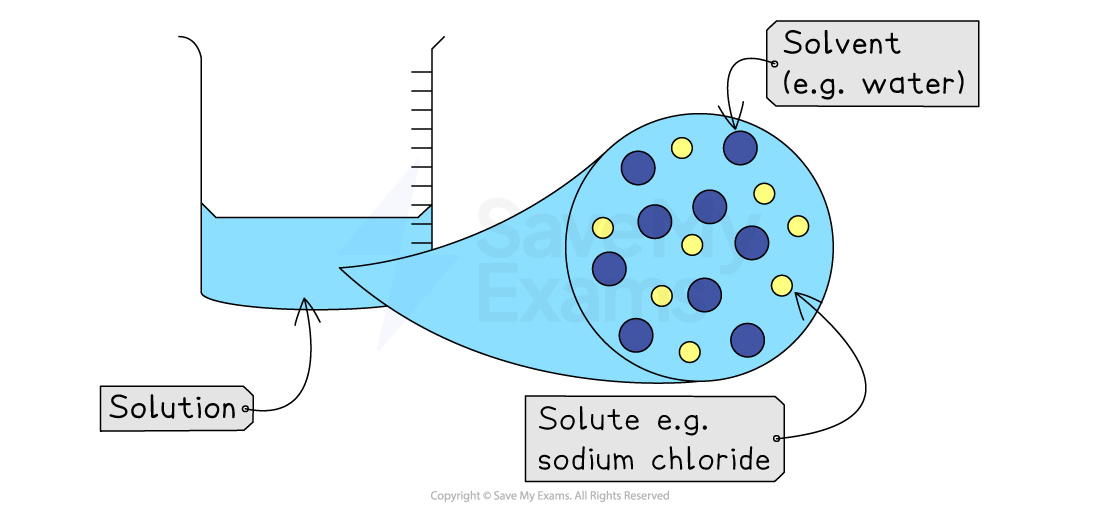
A solution is formed when a substance called the solute dissolves in a liquid called the solvent.
Solute: The substance that dissolves (e.g., sodium chloride)
Solvent: The liquid it dissolves in (e.g., water)
A solution
Concentration is a measure of how much solute is dissolved in a certain volume of solvent
A high concentration means there is a lot of solute
A low concentration (a dilute solution) means there is very little
We measure concentration in moles per litre
The units can be written as mol/l or mol l⁻¹
The concentration triangle
Just like with mass and moles, there is a relationship between the number of moles (n), concentration (c), and volume (v)
We can use a triangle to help with the calculations
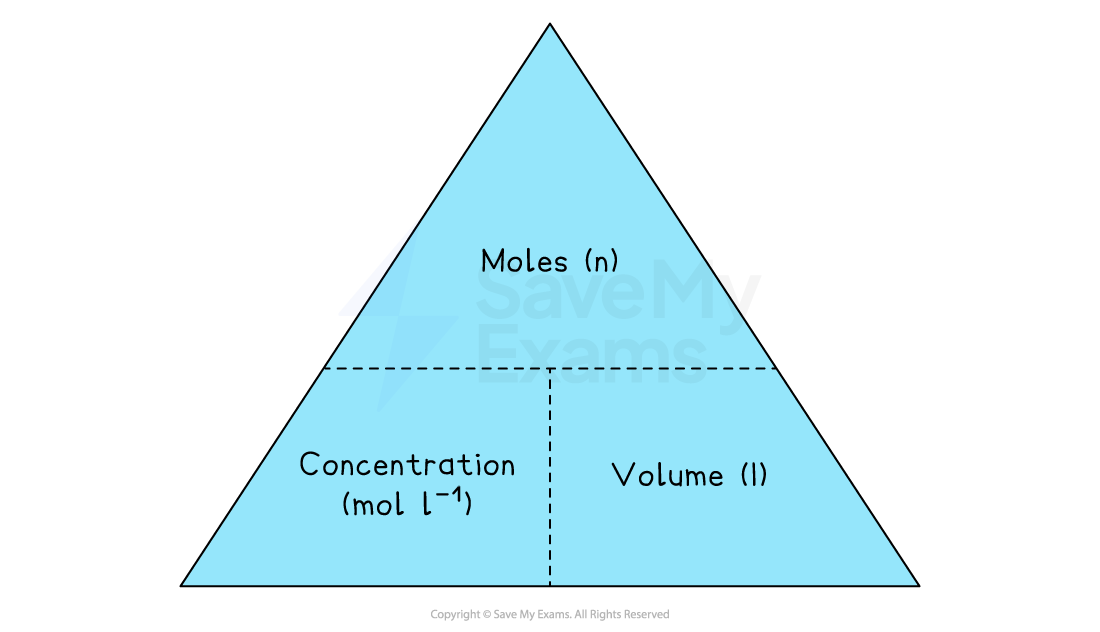
To find moles (n):
Cover n. You are left with C beside V.
n = c × v
To find concentration (c):
Cover c. You are left with n over v.
c = n / v
To find volume (v):
Cover v. You are left with n over c.
v = n / c
Examiner Tips and Tricks
A very common exam mistake is forgetting to convert volumes
In the formula n = c x v, the volume (v) must be in litres.
Questions often give you the volume in millilitres (ml) or cubic centimetres (cm³)
To convert cm³ or ml to litres, you must divide by 1000
500 / 1000 = 0.5 l
25 ml = 25 / 1000 = 0.025 l
Worked Example
Calculating concentration
Calculate the concentration of a solution made by dissolving 10 g of sodium hydroxide, NaOH, in 250 cm³ of water.
[3]
Calculate moles (n):
GFM of NaOH = 23 + 16 + 1 = 40
n = mass / GFM
n = 10 / 40 = 0.25 moles [1 mark]
Convert volume to litres (v):
v = 250 cm³
250 / 1000 = 0.25 l [1 mark]
Calculate concentration (c):
c = n / v
c = 0.25 / 0.25
c = 1 mol l⁻¹ [1 mark]
Worked Example
Calculating mass
What mass of potassium chloride, KCl, is needed to make 500 cm³ of a 0.2 mol l⁻¹ solution?
Convert volume to litres (v):
v = 500 cm³ = 500 / 1000 = 0.5 l [1 mark]
Calculate moles (n):
Use the concentration triangle: n = c x v
n = 0.2 x 0.5 = 0.1 moles [1 mark]
Calculate mass:
GFM of KCl = 39 + 35.5 = 74.5
mass = n x GFM
mass = 0.1 x 74.5
mass = 7.45 g [1 mark]

Unlock more, it's free!
Was this revision note helpful?
