Analytical Methods - Ion Tests (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Flame tests
A flame test is a simple laboratory technique used to identify a metal ion based on the characteristic colour it produces when heated in a Bunsen flame
How to carry out a flame test
Clean a wire loop (usually made of unreactive nichrome) by dipping it in dilute hydrochloric acid
Heat the loop in a roaring blue Bunsen flame until no colour is produced
Dip the clean loop into the solid sample to be tested
Place the end of the loop back into the hot flame and observe the colour
Flame test method

Examiner Tips and Tricks
Before the test, it is essential to clean the wire with acid to remove any contaminating ions from previous tests
If the wire is not clean, the flame colours could mix together, making it impossible to get a clear result
For example, the strong yellow flame of sodium can easily mask other colours
Flame test results
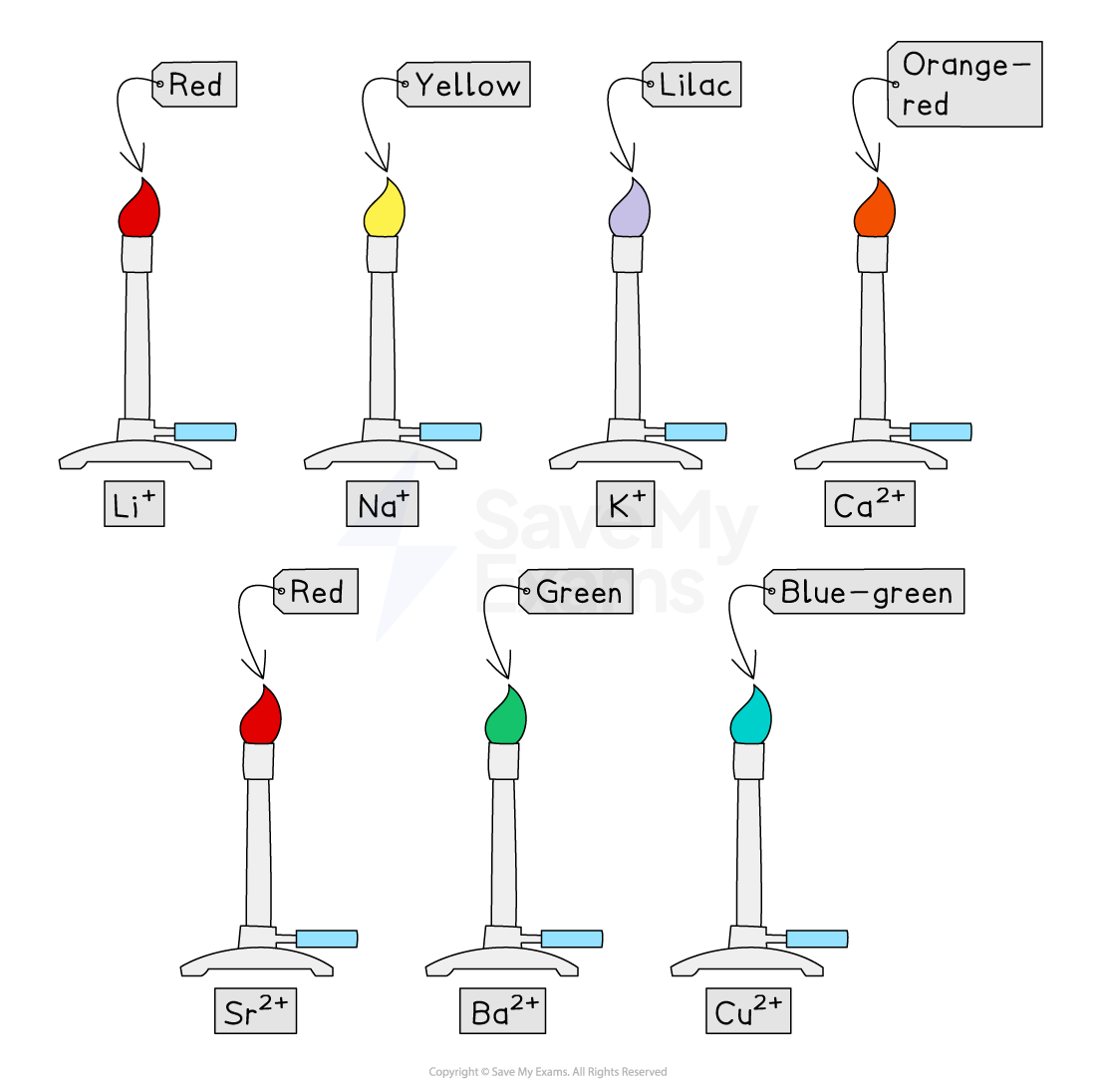
Examiner Tips and Tricks
You do not need to memorise the flame test colours
All the colours you need to know are provided in a table on page 6 of the SQA Data Booklet
Your skill in the exam is finding the correct information in the booklet, not remembering it
Precipitation tests for ions
A precipitation reaction occurs when two different solutions are mixed and they react to form an insoluble solid
This insoluble solid is called a precipitate
Essentially, you are mixing two soluble compounds to create one insoluble one
Predicting when a precipitate will form
To work out if a precipitate will form when two solutions are mixed, you need to know the solubility of the potential new products
The solubility rules for common ionic compounds are given on page 8 of the SQA Data Booklet
You can use this table to predict whether a substance is soluble or insoluble
The method
Identify the ions present in the two starting solutions
Work out the two new "swapped partner" compounds that could form.
Use the table on page 8 of the Data Booklet to look up the solubility of these two new compounds
If one of the new compounds is listed as insoluble (or very slightly soluble), then it will form a precipitate
Using precipitation to identify ions
Precipitation reactions are very useful as chemical tests to identify the presence of specific ions in a solution
If you know that a certain ion forms a distinctively coloured precipitate with a particular reactant, you can use this to test for it.
Testing for chloride ions (Cl-)
Add a few drops of silver nitrate solution (AgNO3 (aq))
The formation of a white precipitate (silver chloride) indicates that chloride ions are present
Testing for sulfate ions (SO42-)
Add a few drops of barium chloride solution (BaCl2 (aq))
The formation of a white precipitate (barium sulfate) indicates that sulfate ions are present
Test for carbonate Ions (CO32-)
Add a small amount of any dilute acid, such as HCl (aq)
Fizzing / effervescence suggests that carbon dioxide gas is produced
This can be confirmed by bubbling it through limewater, which would turn cloudy
For more information about testing for carbon dioxide, see the Gas Tests revision note
Examiner Tips and Tricks
Questions about identifying ions are common in exams
You are expected to know these three standard chemical tests:
Halide ions (Cl-) = silver nitrate solution
Sulfate ions (SO42-) = barium chloride solution
Carbonate ions (CO32-) = dilute acid
Worked Example
A student mixes a solution of potassium chloride with a solution of silver nitrate.
a) State what would be observed.
[1]
b) Write a balanced chemical equation, including state symbols, for the reaction that occurs.
[1]
Answer:
Part a): What would be observed?
Identify the ions present:
Potassium chloride solution: K+ (aq) and Cl- (aq)
Silver nitrate solution: Ag+ (aq) and NO3- (aq)
Work out the new potential products:
The potassium ion (K+) reacts with the nitrate ion (NO3-) to form potassium nitrate
The silver ion (Ag+) reacts with the chloride ion (Cl-) to form silver chloride
Use the Data Booklet (page 8) to check solubility:
Potassium nitrate is soluble, (aq)
Silver chloride is insoluble (s)
Since silver chloride is insoluble, it will form a white precipitate [1 mark]
Part b): To write the balanced symbol equation
Write down the reactants and products formed
KCl + AgNO3 → KNO3 + AgCl
Check atoms are balanced
K : 1 on both sides so K is balanced
Cl: 1 on both sides so Cl is balanced
NO3: 1 on both sides so NO3 is balanced
Ag: 1 on both sides so Ag is balanced
Add in state symbols
Remember that the precipitate must be a solid, therefore the state symbol (s)
KCl (aq) + AgNO3 (aq) → KNO3 (aq) + AgCl (s) [1 mark]

Unlock more, it's free!
Was this revision note helpful?
