Analytical Methods - Titrations (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Titrations
Titration is an accurate experimental method used to determine the exact volume of a solution needed to react completely with another solution
They can also be used to prepare soluble salts
This data can then be used in calculations to find an unknown concentration
Key terminology
Standard solution
A standard solution is one where the concentration is known precisely
In a titration, this is the solution you use to find the concentration of the "unknown" solution
End-point
The end-point is the point at which the reaction is just complete (e.g., the acid has perfectly neutralised the alkali)
It is identified by a sharp, permanent colour change in the indicator.
Titre
The titre is the volume of solution added from the burette to reach the end-point
Apparatus used in a titration

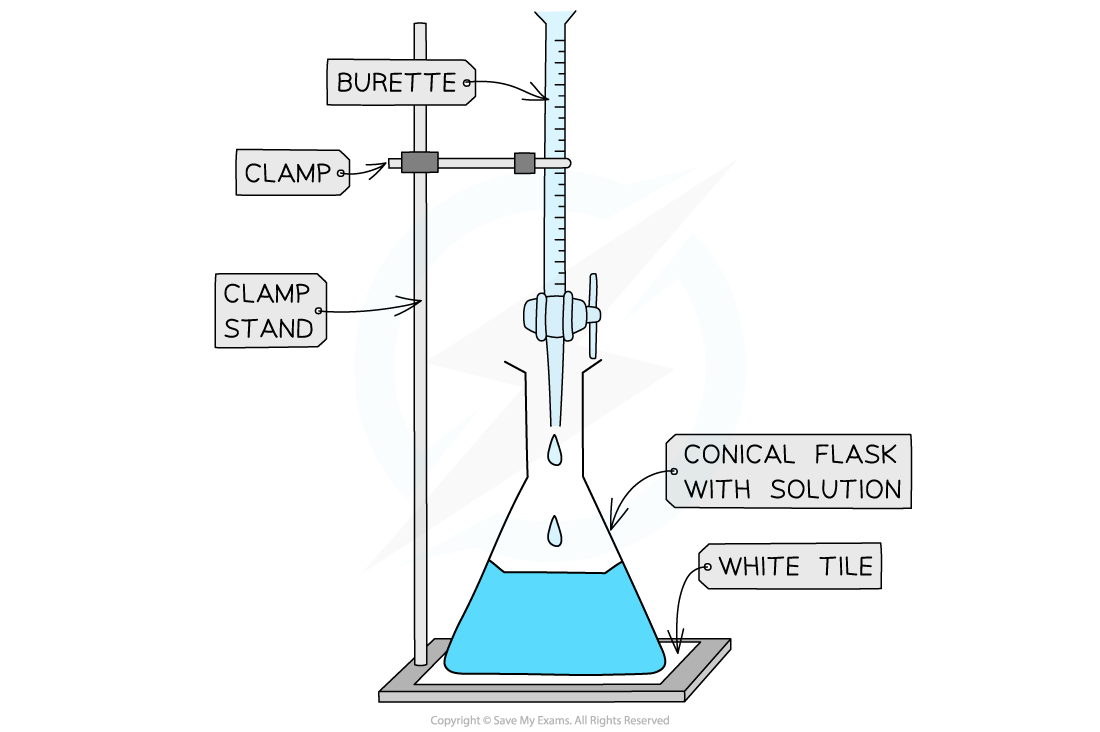
Method
Use the pipette and pipette filler and place exactly 25 cm3 of alkali into the conical flask
Fill the burette with acid, place an empty beaker underneath the tap and run a small portion of acid through the burette to remove any air bubbles
Record the starting point on the burette to the nearest 0.05 cm3
Place the conical flask on a white tile so the tip of the burette is inside the flask
Add a few drops of a suitable indicator to the solution in the conical flask
Perform a rough titration by taking the burette reading and running in the solution in 1 – 3 cm3 portions, while swirling the flask vigorously
Quickly close the tap when the end-point is reached (sharp colour change) and record the volume, placing your eye level with the meniscus
Now repeat the titration with a fresh batch of sodium hydroxide
As the rough end-point volume is approached, add the solution from the burette one drop at a time until the indicator just changes colour
Record the volume to the nearest 0.05 cm3
Repeat until you achieve two concordant results (two results that are within 0.2 cm3 of each other) to increase accuracy
| Rough titre | Titre 1 | Titre 2 |
Final reading (cm3) |
|
| |
First reading (cm3) |
|
|
|
Titre (cm3) |
|
|
|
Practical tip
Use a funnel to fill the burette but be sure to remove it before starting the practical as it can drip liquid into the burette, making the initial reading false
Example results
| Rough titre | Titre 1 | Titre 2 |
Final reading (cm3) | 16.00 | 14.90 | 15.20 |
First reading (cm3) | 0.10 | 0.00 | 0.20 |
Titre (cm3) | 15.90 | 14.90 | 15.00 |
Calculate mean titre using concordant results, 14.90 cm3 and 15.00 cm3
Average titre = (14.90 + 15.00) / 2 = 14.95 cm3
Worked Example
A student's results for a titration are:
Rough = 25.80 cm3
Titre 1 = 25.20 cm3
Titre 2 = 25.10 cm3
What average volume should be used in their calculation?
[1]
Answer:
Step 1: Identify the correct titres to use
The rough titre should be ignored
The results from titre 1 and titre 2 (25.20 cm³ and 25.10 cm³) are concordant as they are within 0.2 cm³ of each other
Step 2: Calculate the average titre:
(25.2 + 25.1) / 2 = 25.15 cm³ [1 mark]

Unlock more, it's free!
Was this revision note helpful?
