Practical Techniques - Calorimetry (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Determining heat energy (Eh)
This practical technique is used to experimentally determine the quantity of heat energy released by a fuel when it undergoes combustion
Equipment
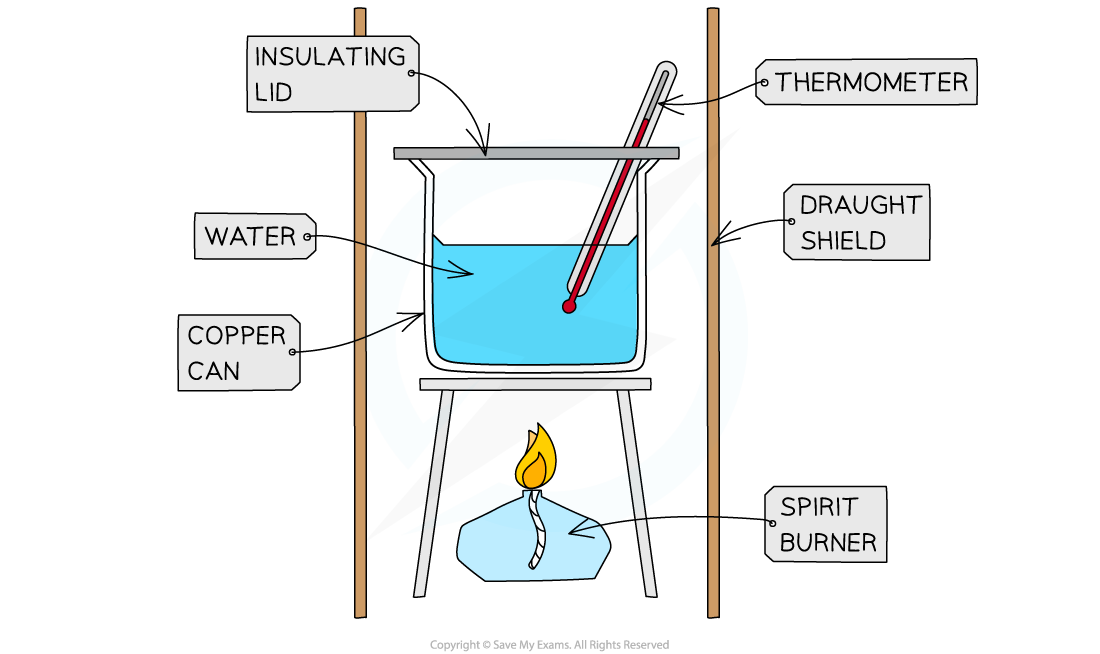
A simple calorimetry experiment uses the following equipment:
A spirit burner containing the fuel
A can or beaker containing a known mass or volume of water
A thermometer to measure the temperature change of the water
A tripod and gauze to support the beaker
A digital balance to measure the mass of the spirit burner before and after the experiment
Draught shields to minimise heat loss to the surroundings
Examiner Tips and Tricks
A common exam question asks how you could make this experiment more accurate. The best answers focus on reducing heat loss:
Use draught shields to protect the flame from drafts
Place a lid on the beaker
Reduce the distance between the flame and the beaker
Use a copper can instead of a glass beaker, as copper is a better conductor of heat
Method
Measure the water
Accurately measure a fixed volume (e.g., 100 cm3) of water into the copper can/beaker.
Record the initial temperature
Place the thermometer in the water and record the starting temperature
Weigh the fuel:
Weigh the spirit burner containing the fuel
Record its initial mass
Heat the water
Place the spirit burner under the beaker and light the wick
Allow it to heat the water until there is a significant temperature rise (e.g., 20-30oC)
Extinguish and record
Extinguish the flame
Record the maximum temperature reached by the water
Reweigh the fuel
Weigh the spirit burner again
Record its final mass
Examiner Tips and Tricks
This simple experiment is very inaccurate because a large amount of heat energy is lost to the surroundings and is not transferred to the water
Key sources of error are:
Heat loss to the surroundings from the beaker and the flame
Incomplete combustion of the fuel, which releases less energy
Heat energy being absorbed by the beaker itself
Because of these errors, the experimental value for the heat energy released is always lower than the true value
Measurements & calculations
From this experiment, you will have recorded:
The mass of water (m)
The initial and final temperatures
This allows you to calculate the temperature change (ΔT)
The initial and final mass of the burner
This allows you to calculate the mass of fuel burned
You can then use the first two measurements in the heat energy equation to find Eh
For full details on how to use this data, see the Calorimetry revision note

Unlock more, it's free!
Was this revision note helpful?
