Practical Techniques - Electrochemistry (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Testing electrical conductivity
This experiment is used to determine whether a substance can conduct electricity
For a substance to conduct, it must contain charged particles that are free to move
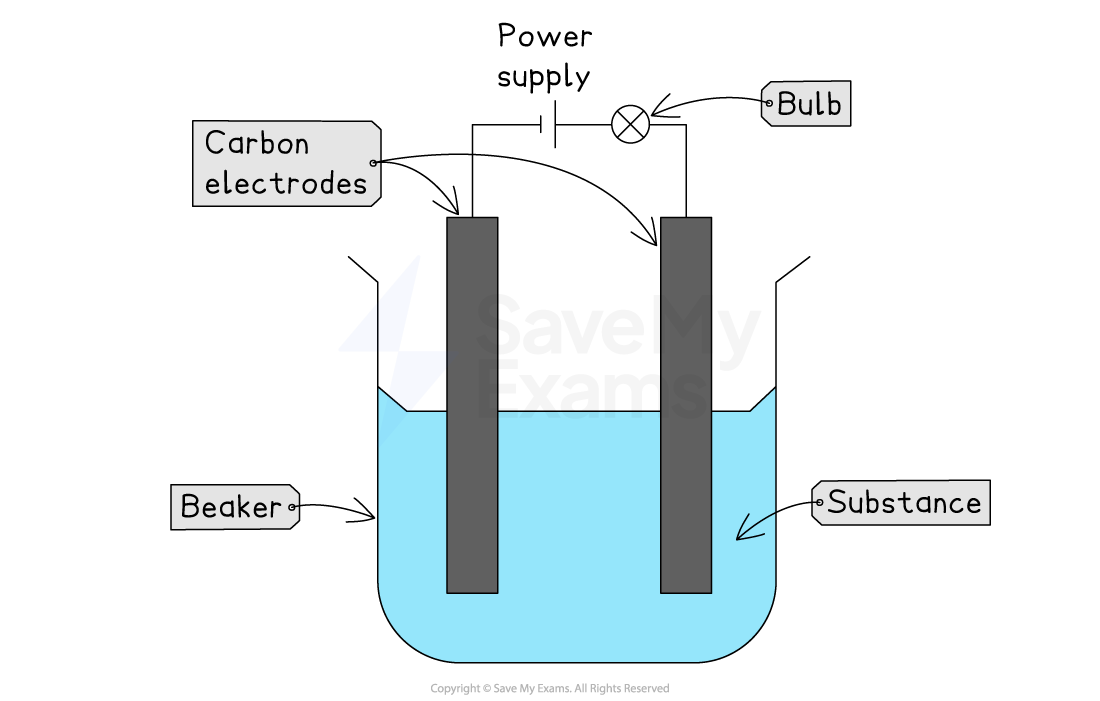
Apparatus
A low-voltage power supply or battery
Two inert (unreactive) electrodes, usually made of graphite (carbon)
A beaker to hold the substance being tested
A lightbulb or ammeter to detect if a current is flowing
Method
Set up the circuit as shown, with the electrodes placed in an empty beaker
Test the substance as a solid
Add the solid to the beaker, ensuring it touches both electrodes
Observe if the bulb lights up
Test the substance as a liquid
If the substance is soluble, add water to create a solution
Observe if the bulb lights up
If the substance is insoluble, safely heat it until it becomes molten
Observe if the bulb lights up
Expected results
The conductivity of a substance depends on its bonding and structure
The results you would expect for the main substance types are summarised below:
Substance | Do they conduct electricity? | Reason |
|---|---|---|
metal | Yes | Contains delocalised electrons that are free to move in any state |
solid ionic compounds | No | Ions are held in a fixed lattice when solid and cannot move |
molten / dissolved ionic compounds | Yes | Ions are free to move when molten or dissolved |
covalent compounds | No - except graphite | Contains neutral molecules and no free-moving charged particles |
Examiner Tips and Tricks
Remember that graphite is the only covalent network that can conduct electricity when solid
This is because it has delocalised electrons that are free to move along its layers
Setting up electrochemical cells
An electrochemical cell (or battery) uses a chemical reaction to produce a voltage
The most common setup involves two separate half-cells connected together
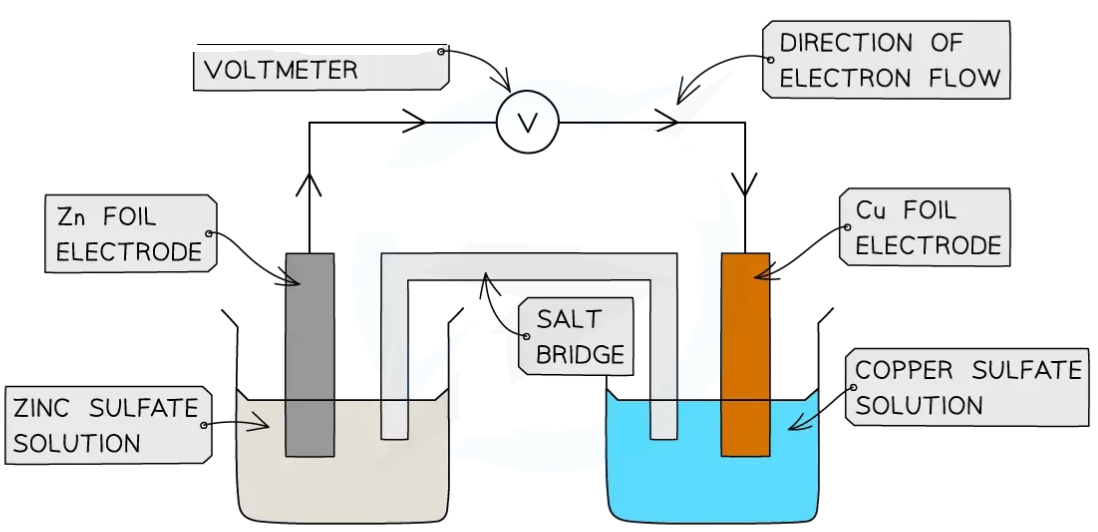
Apparatus
Two beakers
Two different metal electrodes
Solutions of the corresponding metal ions
A voltmeter
Wires and crocodile clips
An ion bridge (filter paper soaked in an electrolyte like potassium nitrate)
Method
Create half-cell 1:
Place a metal rod into a beaker containing a solution of its own ions
For example, place a zinc rod into a beaker of zinc sulfate solution
Create half-cell 2:
Do the same for a different metal
For example, place a copper rod into a beaker of copper(II) sulfate solution
Connect the electrodes:
Use crocodile clips and wires to connect the two metal rods (the electrodes) to a voltmeter
Complete the circuit:
Soak a strip of filter paper in a neutral ionic solution (like potassium nitrate) and use it to connect the two beakers
This is the ion bridge
Observe the voltage produced on the voltmeter
Practical tip
If one of the half-cells does not contain a metal (for example, an iodine/iodide solution), a graphite (carbon) rod should be used as the inert electrode in that half-cell
Solution electrolysis
Electrolysis is the process of using electricity to break down an ionic compound
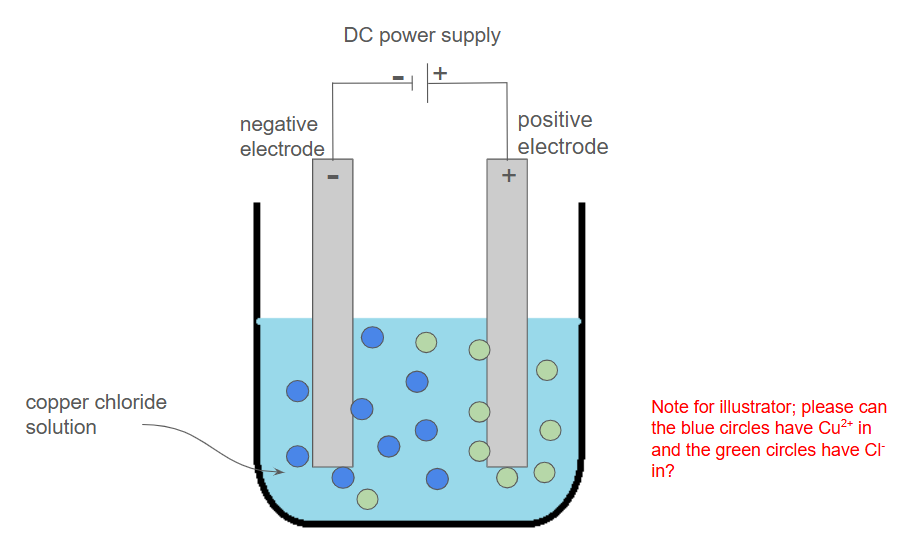
Apparatus
A beaker to hold the electrolyte
An ionic solution (the electrolyte)
Two inert graphite electrodes
A DC (direct current) power supply
Wires and crocodile clips
Method
Prepare the electrolyte
Pour the ionic solution to be tested (the electrolyte, e.g., copper(II) chloride solution) into a beaker
Set up the electrodes
Place two inert graphite electrodes into the solution, making sure they do not touch
Connect the power supply
Use wires to connect the electrodes to a DC (direct current) power supply
It is crucial to use a DC supply so the electrodes are fixed as positive and negative
Switch on the power supply
Observe the chemical changes that occur at each electrode
For example, bubbles of gas, solid forming

Unlock more, it's free!
Was this revision note helpful?
