Practical Techniques - Salt Preparation (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Preparation of soluble salts
What is a salt?
A salt is a compound that is formed when the hydrogen atom in an acid is replaced by a metal
For example, if we replace the H in HCl with a potassium atom, then the salt potassium chloride is formed, KCl
How to make a soluble salt
There are two options for making soluble salts
The method you choose depends on whether your starting base is soluble or insoluble
Option 1: Using an insoluble base
This method is used to make a soluble salt from an insoluble base, such as:
An insoluble metal oxide
An insoluble metal hydroxide
An insoluble metal carbonate
Method:
React
Gently warm the dilute acid and add the insoluble base in excess (add it until no more reacts and you can see some unreacted solid).
Filter
Filter the mixture to remove the unreacted excess solid base.
The liquid that passes through (the filtrate) is your pure salt solution.
Evaporate and crystallise
Gently heat the filtrate in an evaporating basin to evaporate some of the water.
Once crystals start to form, leave the solution to cool and crystallise slowly.
Dry
Remove the crystals and pat them dry.
For full details, see the Producing Soluble Salts from Carbonates or Oxides revision note
Example: preparation of pure, hydrated copper(II) sulfate crystals
Acid = dilute sulfuric acid
Insoluble base = copper(II) oxide
Method
Add dilute sulfuric acid into a beaker and heat using a Bunsen burner flame
Add copper(II) oxide (insoluble base), a little at a time to the warm dilute sulfuric acid and stir until the copper(II) oxide is in excess (stops disappearing)
To check the acid has been neutralised touch the glass rod onto indicator paper
Filter the mixture into an evaporating basin to remove the excess copper(II) oxide
Heat the solution to evaporate half of the water and to make the solution saturated.
Check the solution is saturated by dipping a cold, glass rod into the solution and seeing if crystals form on the end
Leave the filtrate in a warm place to dry and crystallise
Decant excess solution and allow crystals to dry or blot to dry with filter paper
Equation of reaction:
copper(II) oxide + sulfuric acid → copper(II) sulphate + water
CuO (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l)
Practical tip
Allowing the filtered solution to evaporate slowly over about a week will result in the formation of larger crystals
Heating the filtered solution will result in the formation of smaller crystals
Worked Example
A student prepared copper(II) sulfate crystals using copper(II) carbonate and sulfuric acid.
a) Write the balanced symbol equation for this reacting including state symbols.
b) Explain why the student add the base in excess.
c) Explain why the student filtered the solution.
d) Describe how could the student could form salt crystals.
Answer:
a) CuCO3 (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l) + CO2 (g)
b) The student added the base in excess to ensure that all the acid had reacted
c) The student filtered the solution to remove any unreacted copper(II) carbonate
d) To form dry salt crystals the student should transfer the solution to an evaporating dish, heat strongly with a Bunsen burner until half of the water has evaporated and then transfer to a warm oven for crystals to form. Finally decant excess solution and allow crystals to dry or blot to dry with filter paper.
Option 2: Using a soluble base / alkali
This method is used to make a soluble salt from a soluble base
For example, the alkali sodium hydroxide
Method:
Titrate
Perform a titration using an indicator to find the exact volume of acid needed to neutralise a fixed volume of the alkali.
Repeat without indicator
Repeat the experiment using the exact same volumes of acid and alkali, but without the indicator
This creates a pure, uncontaminated salt solution.
Evaporate and crystallise
Gently evaporate the water from the pure salt solution and allow crystals to form, as in Option 1
For full details, see the Producing Soluble Salts by Titration revision note
Preparation of insoluble salts
Insoluble salts can be prepared using a precipitation reaction
The solid salt obtained is the precipitate, thus in order to successfully use this method the solid salt being formed must be insoluble in water, and the reactants must be soluble
Using two soluble reactants to prepare an insoluble salt
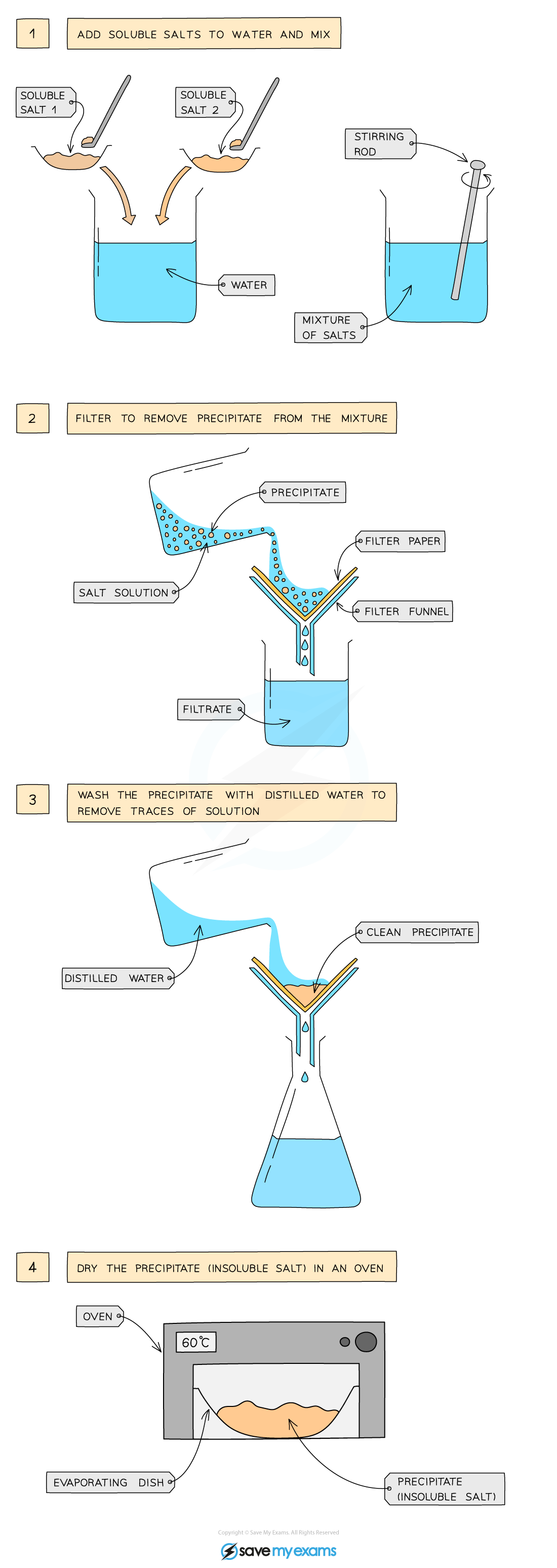
Method
Dissolve soluble salts in water and mix together using a stirring rod in a beaker
Filter to remove precipitate from mixture
Wash residue with distilled water to remove traces of other solutions
Leave in an oven to dry
Example: Preparation of pure, dry lead(II) sulfate crystals using a precipitation reaction
Soluble salt 1 = lead(II) nitrate
Soluble salt 2 = potassium sulfate
Method
Dissolve lead(II) nitrate and potassium sulfate in water and mix together using a stirring rod in a beaker
Filter to remove precipitate from mixture
Wash precipitate with distilled water to remove traces of potassium nitrate solution
Leave in an oven to dry
Equation of reaction
lead(II) nitrate + potassium sulfate → lead(II) sulfate + potassium nitrate
Pb(NO3)2 (aq) + K2SO4 (aq) → PbSO4 (s) + 2KNO3 (aq)
Examiner Tips and Tricks
Use the Data Booklet (page 8) to check solubility of salts

Unlock more, it's free!
Was this revision note helpful?
