Electrochemical Cells (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Building electrochemical cells
An electrochemical cell (or just a 'cell') is a device that uses a chemical reaction to produce electricity.
It is a way of converting chemical energy into electrical energy
A cell is created by connecting two different conducting materials in a solution
For National 5 Chemistry, there are three cells you need to know about:
A simple cell
The half cell-system
Non-metal half-cells
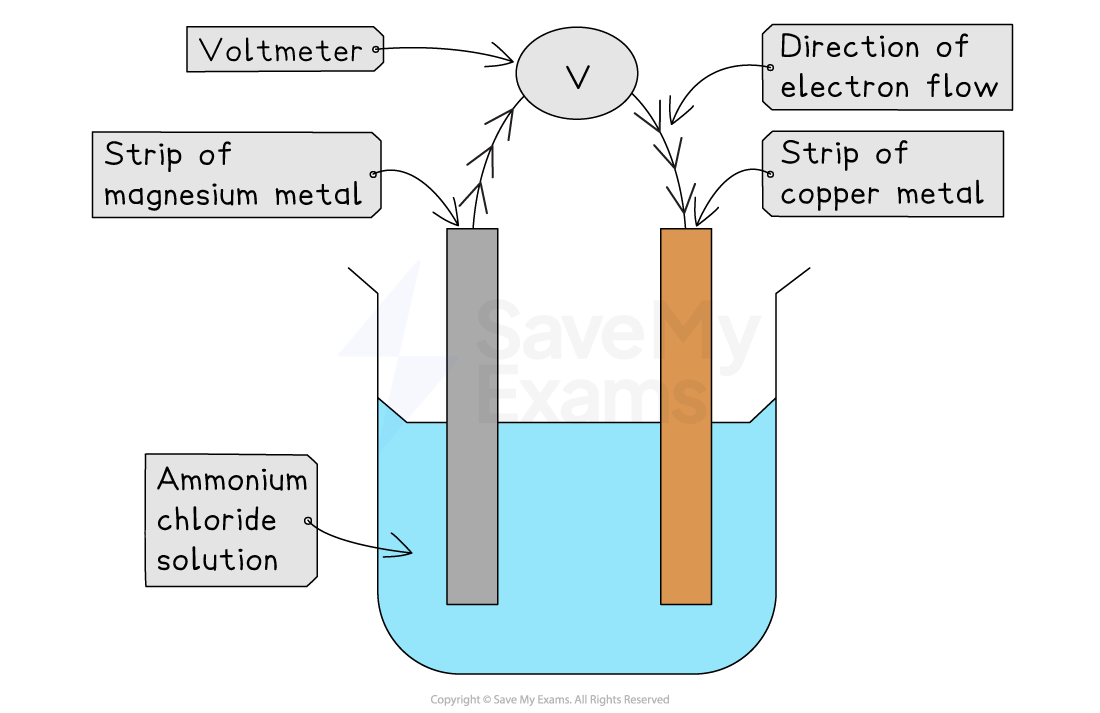
A simple cell
The simplest type of cell can be made by placing two different metals into an electrolyte
An electrolyte is a solution that can conduct electricity because it contains free-moving ions
Ionic solutions, like salt water or ammonium chloride solution, are electrolytes
The two different metals are connected by a wire through a voltmeter
When the metals are dipped into the electrolyte, a voltage is produced and a current flows
Example simple cell
The half cell-system
To study the reactions happening in a cell more closely, chemists often set it up using two separate beakers, called half-cells
A half-cell consists of a metal rod placed in a solution containing its own ions
For example, a copper rod in a solution of copper(II) sulfate
To make a full cell, two different half-cells are connected:
A wire and voltmeter connects the two metal rods, allowing electrons to flow
An ion bridge (or salt bridge) connects the two solutions
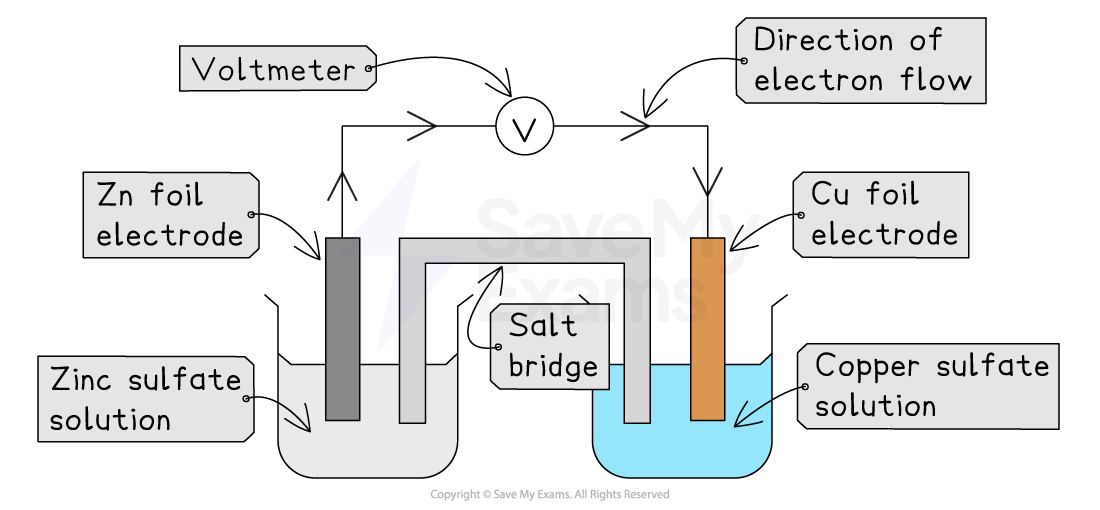
The ion bridge
The ion bridge is essential for the cell to work
It is usually a piece of filter paper or a tube filled with an ionic solution (like potassium nitrate)
The ion bridge completes the circuit by allowing ions to move between the two half-cells
This prevents a build-up of charge in either beaker, which would stop the flow of electricity
Non-metal half-cells
Electricity can be produced even if one of the half-cells does not contain a metal
For example, a half-cell could be made with a solution of iodine and iodide ions
Since there is no metal rod to act as an electrical conductor, a different material must be used for the electrode.
In half-cells that do not contain a metal, a graphite rod is used as the electrode.
Graphite is chosen because it is unreactive and conducts electricity
A cell can be constructed using a zinc half-cell and an iodine half-cell
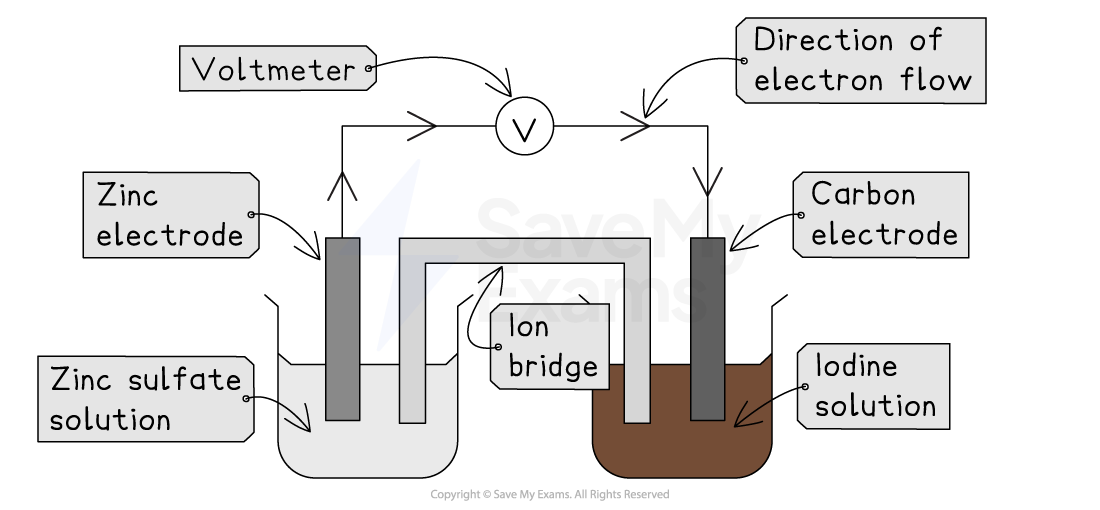
Half-cell 1:
A zinc metal rod placed in a solution of zinc sulfate (containing Zn2+ ions)
Half-cell 2:
A carbon rod placed in a solution containing both iodine and potassium iodide (containing I2 molecules and I- ions)
The two half-cells are connected by a wire and voltmeter, and an ion bridge
How to determine the overall reaction
Find them in the electrochemical series (data booklet, page 10):
Zinc (Zn) is very high up the series
Iodine (I2) is much lower down, below copper
Determine electron flow:
Electrons always flow from the species higher up the series to the one lower down
Therefore, electrons flow from the zinc rod to the carbon rod
Examiner Tips and Tricks
A very common mistake is to confuse what moves where
Remember:
Electrons are subatomic particles that flow through the external wires and the voltmeter
Ions are charged particles in the solution that move through the ion bridge to complete the circuit
So, in this example, electrons flow from the zinc rod, through the wire, to the carbon rod
Write the ion-electron equations:
At the zinc electrode (oxidation):
Zinc is higher, so its equation is reversed
The zinc metal loses electrons and the rod will get smaller
Zn (s) → Zn2+ (aq) + 2e-
At the carbon electrode (reduction):
Iodine is lower, so its equation is used as written.
The carbon electrode simply provides a surface for the iodine molecules to gain electrons and turn into iodide ions
I2 (aq) + 2e- → 2I- (aq)
This complete circuit produces a voltage, demonstrating that a cell can function perfectly well with a non-metal half-cell

Unlock more, it's free!
Was this revision note helpful?
