Extraction of Metals (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Methods of metal extraction
Most metals are found in the Earth's crust not as pure elements, but chemically bonded to other elements in compounds
A rock that contains enough of a metal compound to be worth extracting is called an ore
Extraction of metals
To get the pure metal, it must be extracted from its ore
In the ore, the metal exists as a positive ion
The extraction of a metal from its ore is always a reduction reaction
The positive metal ions must gain electrons to become neutral metal atoms
For example:
Al3+ + 3e- → Al
The method used for extraction depends entirely on the metal's reactivity
Very reactive metals form very stable compounds, which are difficult to break down, and therefore require more energy to extract
Extraction by heat
This method is suitable for the least reactive metals, such as silver, gold, and mercury
The compounds of these metals are unstable and easily decompose
Heating the ore provides enough energy to break the bonds and release the metal
For example, the extraction of silver from silver oxide:
silver oxide → silver + oxygen
2Ag2O (s) → 4Ag (s) + O2 (g)
Each silver ion (Ag+) gains one electron:
Ag+ + e- → Ag
Extraction by heating with carbon (or carbon monoxide)
This method is suitable for metals in the middle of the electrochemical series, such as copper, lead, tin, iron, and zinc
These metals are too reactive to be extracted by heat alone
They must be heated in a furnace with a reducing agent
A reducing agent is a substance that removes oxygen
Carbon (in the form of coke) or carbon monoxide (CO) are commonly used reducing agents
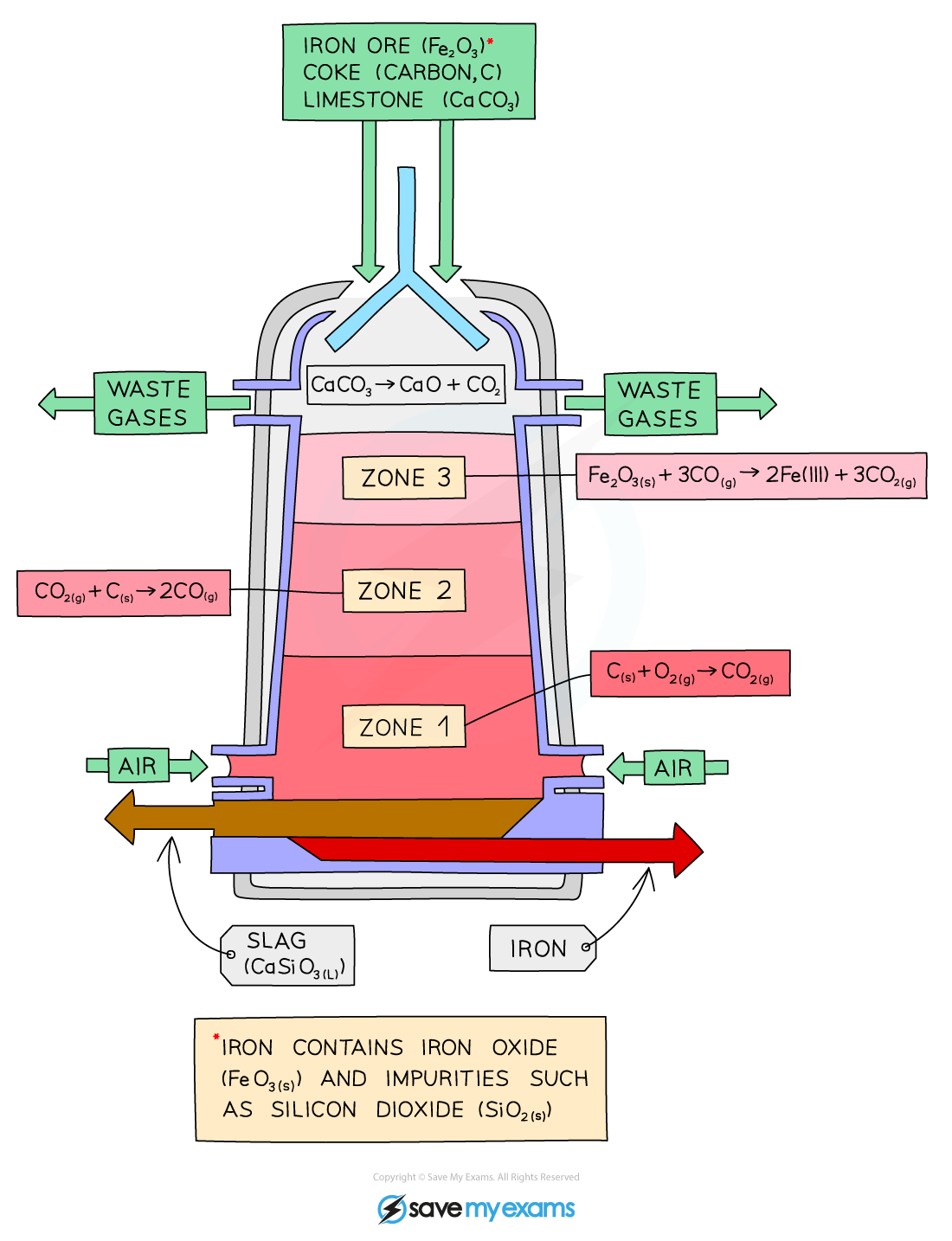
For example, the extraction of iron in a blast furnace:
iron(III) oxide + carbon monoxide → iron + carbon dioxide
Fe2O3 (s) + 3CO (g) → 2Fe (l) + 3CO2 (g)
Each iron(III) ion (Fe3+) gains three electrons:
Fe3+ + 3e- → Fe
The blast furnace
Extraction by electrolysis
This method is suitable for the most reactive metals, such as potassium, sodium, calcium, magnesium, and aluminium
These metals form very stable compounds that cannot be reduced by carbon
Electrolysis, which uses a powerful direct electric current to break down the compound, is required
This process is very expensive due to the high energy demand
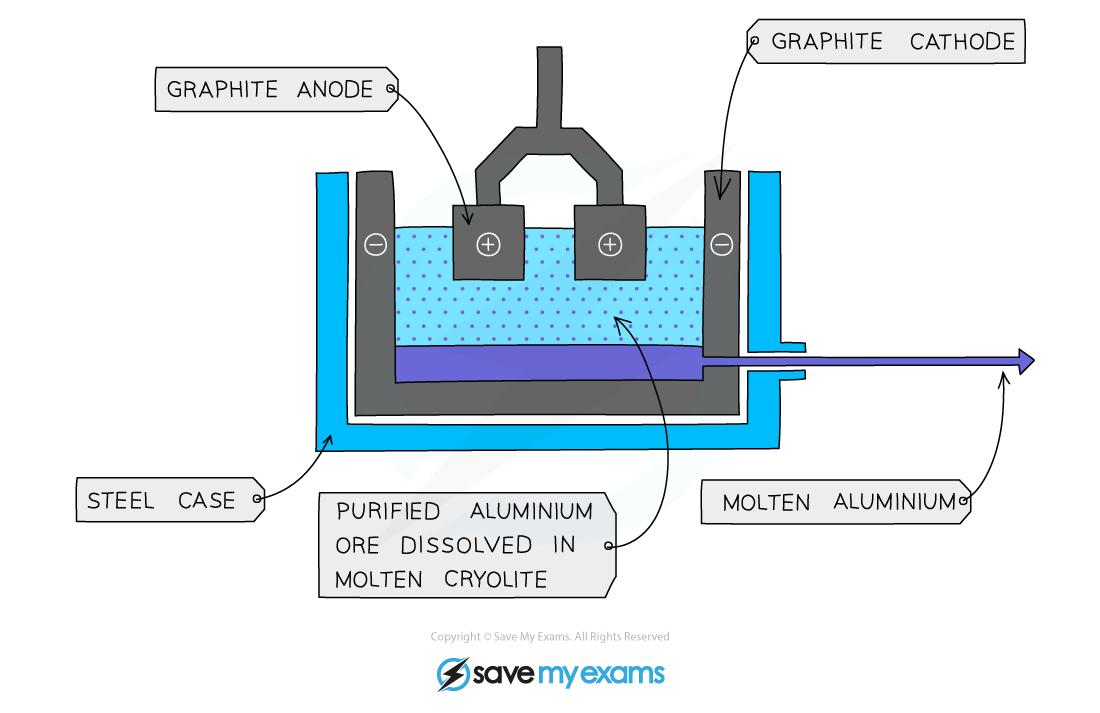
For example, the extraction of aluminium from aluminium oxide:
The molten aluminium ore is decomposed by electricity
The positive aluminium ions (Al3+) are attracted to the negative electrode
At the negative electrode, each aluminium ion gains three electrons to become a neutral aluminium atom:
Al3+ + 3e- → Al
Extraction of aluminium
Extraction summary
Position in the electrochemical series | Example metals | Method of extraction |
|---|---|---|
High (most reactive) | K, Na, Ca, Mg, Al | Electrolysis |
Middle | Zn, Fe, Pb, Cu | Heating with carbon / carbon monoxide |
Low (least reactive) | Ag, Au, Hg | Heat alone |

Unlock more, it's free!
Was this revision note helpful?
