Metallic Bonding (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Metallic bonding & conductivity
Metallic bonding is the force of attraction that holds the atoms together in a metallic element
It is found in all metals and alloys
A metallic bond is the strong electrostatic force of attraction between the positively charged ions and the delocalised electrons
Metallic bonding
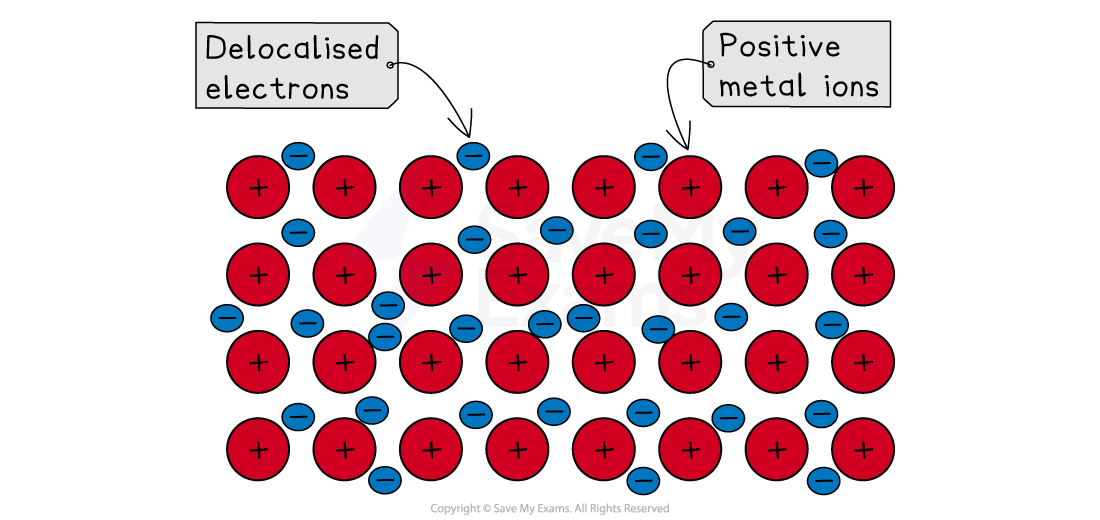
Sea of delocalised electrons
Metal atoms have a weak hold on their outer shell electrons
In a metallic structure, these outer electrons are lost from the atoms and become delocalised
These are electrons that are no longer associated with any single atom
They are free to move throughout the entire metal structure
When the atoms lose these electrons, they become positively charged ions
These positive ions are arranged in a regular, repeating pattern, forming a giant lattice
The delocalised electrons flow around and between these positive ions, like a "sea" of electrons
Electrical conductivity
The unique structure of metals explains their properties
The most important one to explain is electrical conductivity
Metals are excellent conductors of electricity in both the solid and liquid states
To conduct electricity, a substance needs charged particles that are free to move
Metals contain delocalised electrons
These electrons are free to move throughout the metallic lattice
When a voltage is applied, the delocalised electrons are forced to move towards the positive terminal, creating a flow of charge, which is an electric current

Examiner Tips and Tricks
It is essential to use the correct terminology
When explaining why metals conduct electricity, you must state:
They contain "delocalised electrons"...
...which are "free to move"
Just saying "they have free electrons" is not enough to gain the mark

Unlock more, it's free!
Was this revision note helpful?
