Reactions of Metals (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Metal reactivity series
Some metals, like potassium, are extremely reactive, while others, like gold, are very unreactive
By comparing how vigorously different metals react, we can arrange them in order of their reactivity
This ordered list is called the reactivity series
For National 5 Chemistry, the official version of this is the Electrochemical Series
This can be found on page 10 of the SQA Data Booklet
Metals higher up the series are more reactive
Building a simple reactivity series
In the lab, a simple reactivity series can be built by comparing the the rate (speed) of reaction for different metals with the same substance, such as dilute acid
Method:
Add small, equal-sized pieces of different metals to separate test tubes containing the same dilute acid
The reaction produces hydrogen gas, which is observed as fizzing (effervescence)
Observation:
The speed of the fizzing indicates the reactivity
The faster the fizzing, the more reactive the metal
Metal | Observation with acid | Reactivity |
|---|---|---|
Magnesium | Fizzes very vigorously | Very reactive |
Zinc | Fizzes steadily | Reactive |
Iron | Fizzes very slowly | Slightly reactive |
Copper | No reaction (no fizzing) | Unreactive |
From these observations, we can create a simple reactivity series for these four metals:
most reactive > least reactive
magnesium > zinc > iron > copper
This order matches their positions in the full Electrochemical Series
Examiner Tips and Tricks
When describing an experiment to compare reactivity, be specific about what you are observing
Poor answer: Magnesium was more reactive than zinc.
Good answer: Magnesium reacted faster with the acid than zinc did, as it produced bubbles of gas at a greater rate.
Metal reactions
Reactive metals take part in chemical reactions where the metal atoms lose electrons (are oxidised) to form compounds
For National 5 Chemistry, you need to know the general word equations for the reactions of metals with:
Oxygen
Water
Dilute acids
Reaction with oxygen
This reaction happens when a metal is burned in air or oxygen.
The product is a metal oxide
The general word equation is:
metal + oxygen → metal oxide
Worked Example
Write the word and balanced symbol equation for the reaction between calcium and oxygen.
[2]
Answer:
Word equation:
The only product is a metal oxide, so it must be calcium oxide
calcium + oxygen → calcium oxide [1 mark]
Balanced formula equation:
For calcium oxide, Ca is in Group 2 (valency 2) and O is in Group 6 (valency 2)
This gives the formula CaO
Ca + O2 → CaO
The oxygen atoms don't balance (2 on the left, 1 on the right)
Put a 2 in front of CaO to fix this:
Ca + O2 → 2CaO
Now the calcium atoms don't balance (1 on the left, 2 on the right)
Put a 2 in front of Ca to fix this and add state symbols
2Ca (s) + O2 (g) → 2CaO (s) [1 mark]
Reaction with water
Only the most reactive metals will react with cold water
These include the alkali metals like lithium, sodium and potassium
The products are a metal hydroxide and hydrogen gas
The general word equation is:
metal + water → metal hydroxide + hydrogen
Worked Example
Write the word and balanced symbol equation for the reaction between lithium and water.
[2]
Answer:
Word equation:
The products are a metal hydroxide and hydrogen gas
The metal hydroxide must be lithium hydroxide
lithium + water → lithium hydroxide + hydrogen [1 mark]
Balanced formula equation:
For lithium hydroxide, Li is in Group 1 (valency 1). Hydroxide is a group ion, OH⁻ (valency 1)
The formula is LiOH
Li + H2O → LiOH + H2
The hydrogen atoms don't balance (2 on the left, 3 on the right).
A useful trick is to put a 2 in front of the term with the odd number of hydrogens,
Li + H2O → 2LiOH + H2
This gives 4 H on the right, so we need 4 on the left.
Put a 2 in front of H2O:
Li + 2H2O → 2LiOH + H2
Now balance the Li by putting a 2 in front of it and adding state symbols
2Li (s) + 2H2O (l) → 2LiOH (aq) + H2 (g) [1 mark]
Reaction with acid
Metals that are above hydrogen in the Electrochemical Series will react with dilute acids
The products are a salt and hydrogen gas
The general word equation is:
metal + acid → salt + hydrogen
Worked Example
Write the word and balanced symbol equation for the reaction between magnesium and nitric acid.
[2]
Answer:
Word equation:
As nitric acid is used a nitrate salt will be produced
The products are a magnesium nitrate and hydrogen gas
magnesium + nitric acid → magnesium nitrate + hydrogen [1 mark]
Balanced formula equation:
The formulas are:
Magnesium is Mg
Nitric acid is HNO3
Hydrogen is H2
For magnesium nitrate, Mg is in Group 2 (valency 2)
Nitrate is a group ion, NO3- (valency 1)
Swapping valencies and using brackets gives the formula Mg(NO3)2
Mg + HNO3 → Mg(NO3)2 + H2
The nitrate group is not balanced (1 on the left, 2 on the right)
Put a 2 in front of HNO3 to fix this:
Mg + 2HNO3 → Mg(NO3)2 + H₂
Now check all the atoms: 1 Mg, 2 H, 2 N, 6 O on both sides
Mg (s) + 2HNO3 (aq) → Mg(NO3)2 (aq) + H2 (g) [1 mark]
Examiner Tips and Tricks
Both the reaction with water and the reaction with acid produce hydrogen gas
The hydrogen gas produced can be detected using the "pop test"
A lit splint is placed at the mouth of the test tube and will go out with a squeaky 'pop' if hydrogen is present
Using metals to make soluble salts
This method is used to make a soluble salt from the reaction of a moderately reactive metal and a dilute acid
Suitable metals are:
Above hydrogen in the Electrochemical Series
But, not so reactive that the reaction is dangerous
For example, metals like magnesium, zinc or iron).
Unreactive metals like copper (below hydrogen) will not react
The "excess" method
This practical procedure is another example of the "excess method"
The principle is to add more solid reactant (the metal) than is needed
This ensures that all of the acid is completely used up
This also stops the final salt being impure because it is contaminated with unreacted acid
Any leftover solid can be easily removed by filtration
The method in brief
React
Add the metal in excess to a warm dilute acid
The reaction is complete when the fizzing stops and there is unreacted metal left over
Filter
Filter the mixture to remove the unreacted excess metal
The liquid that passes through is the pure salt solution (filtrate)
Crystallise
Gently evaporate the water from the filtrate and allow pure salt crystals to form
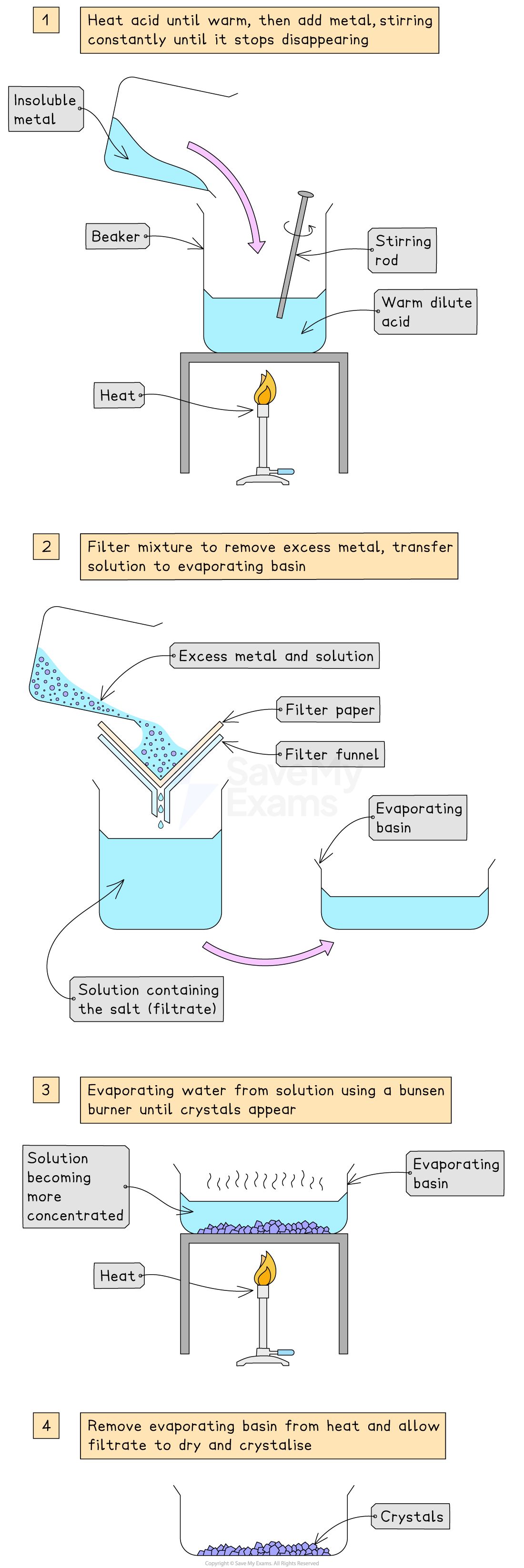
This is the same practical procedure as preparing a salt from an insoluble oxide or carbonate
For a full step-by-step guide and diagrams, see the Preparation of Soluble Salts From Metal Oxides or Carbonates revision note
Examiner Tips and Tricks
You must be able to explain the key steps:
Why add the metal in excess?
To ensure all of the acid is neutralised/reacted
Why filter the mixture?
To remove the unreacted/excess metal

Unlock more, it's free!
Was this revision note helpful?
