Nuclear Equations (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Balancing nuclear equations
Nuclear equations use nuclide notation to show the changes in an atomic nucleus during radioactive decay
Particle | Composition | Nuclide Notation |
|---|---|---|
alpha (α) | 2 protons, 2 neutrons | |
beta (β) | electron from nucleus | |
proton | proton | |
neutron | neutron |
The rules of balancing nuclear equations
A nuclear equation is balanced when the totals on both sides of the arrow are equal
There are two rules:
Conservation of mass number:
The sum of the mass numbers (top numbers) on the left side must equal the sum of the mass numbers on the right side
Conservation of atomic number:
The sum of the atomic numbers (bottom numbers) on the left side must equal the sum of the atomic numbers on the right side
Alpha (α) decay nuclear equations
During alpha decay, an alpha particle is emitted from the unstable nucleus
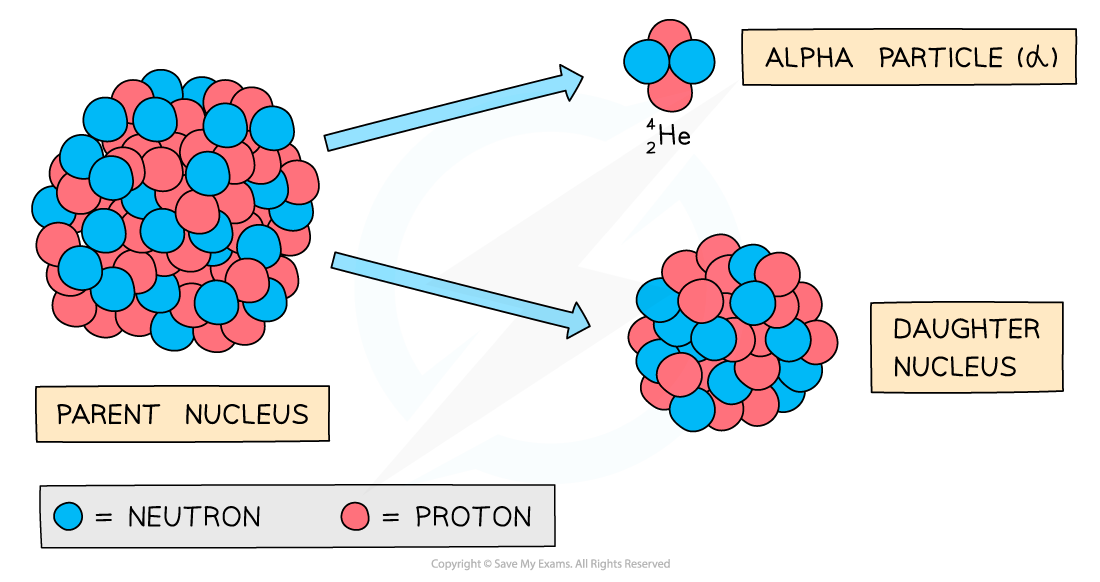
An alpha particle has a mass number of 4
This means that the mass number of the decaying nucleus decreases by 4
An alpha particle has an atomic number of 2
This means that the atomic number of the decaying nucleus decreases by 2
Since the atomic number decreases by 2, a new element is formed
For example, the nuclear equation for polonium-212 undergoing alpha decay to form lead-208 is:
→
+
Worked Example
Radium-226 () undergoes alpha decay. Write the balanced nuclear equation for this process.
[1]
Answer:
Write the starting radioisotope on the left and the emitted alpha particle on the right
→ ? +
Balance the mass numbers (top):
Left-hand side = 226
So, the right-hand side must equal 226
Mass number of unknown = 226 - 4 = 222
Balance the atomic numbers (bottom):
Left-hand side = 88
So, the right-hand side must equal 88
Atomic number of unknown = 88 - 2 = 86
Identify the new element:
Use the Data Booklet to find the element with atomic number 86
This is radon (Rn)
Write the final balanced equation:
→
+
[1 mark]
Beta (β) decay nuclear equations
During beta decay, a neutron changes into a proton and an electron inside the nucleus
The electron is emitted from the nucleus
The proton remains in the nucleus
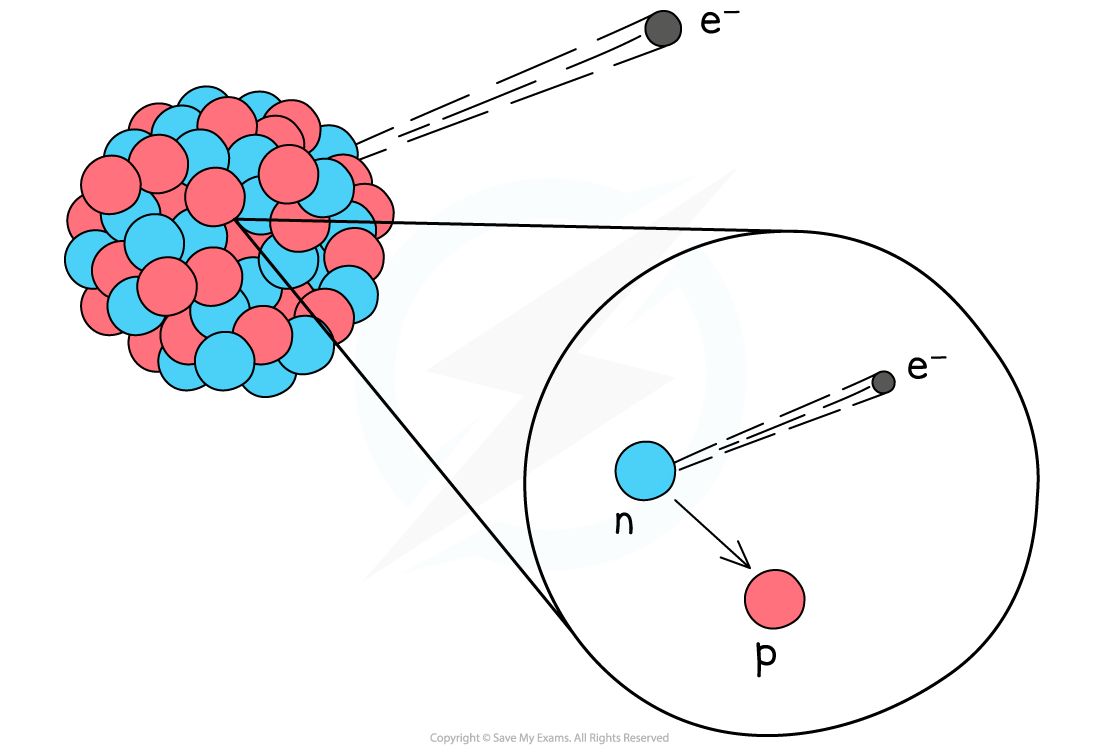
A beta particle has a mass number of 0
This means that the mass number of the decaying nucleus stays the same
A beta particle has an atomic number of -1
This means that the atomic number of the decaying nucleus increases by 1
Since the atomic number increases by 1, a new element is formed
For example, the nuclear equation for carbon-14 undergoing beta decay is:
→
+
Worked Example
Iodine-131 () undergoes beta decay. Write the balanced nuclear equation for this process.
[1]
Answer:
Write the starting radioisotope on the left and the emitted beta particle on the right
→ ? +
Balance the mass numbers (top):
Left-hand side = 131
So, the right-hand side must equal 131
Mass number of unknown = 131 - 0 = 131
Balance the atomic numbers (bottom):
Left-hand side = 53
So, the right-hand side must equal 53
Atomic number of unknown = 53 - (-1) = 54
Identify the new element:
Use the Data Booklet to find the element with atomic number 54
This is xenon (Xe)
Write the final balanced equation:
→
+
[1 mark]
Gamma (γ) decay nuclear equations
During gamma decay, a gamma ray is emitted from an unstable nucleus

The gamma ray has no mass or charge
This means that there is no change to the atomic number or mass number
For example, the nuclear equation for uranium-238 undergoing gamma decay is:
→
+ γ
Examiner Tips and Tricks
Questions on balancing nuclear equations in the exam will almost always focus on alpha and beta decay
This is because they are the nuclear equations where the numbers change

Unlock more, it's free!
Was this revision note helpful?
