Calorimetry (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Calorimetry experiments
Different fuels release different quantities of energy when they burn
The amount of heat energy released by a fuel can be measured using a technique called calorimetry
A simple calorimetry experiment involves using the heat from a burning fuel to raise the temperature of a known mass of water
The full practical details of this experiment are covered in the Practical Techniques for Calorimetry revision note
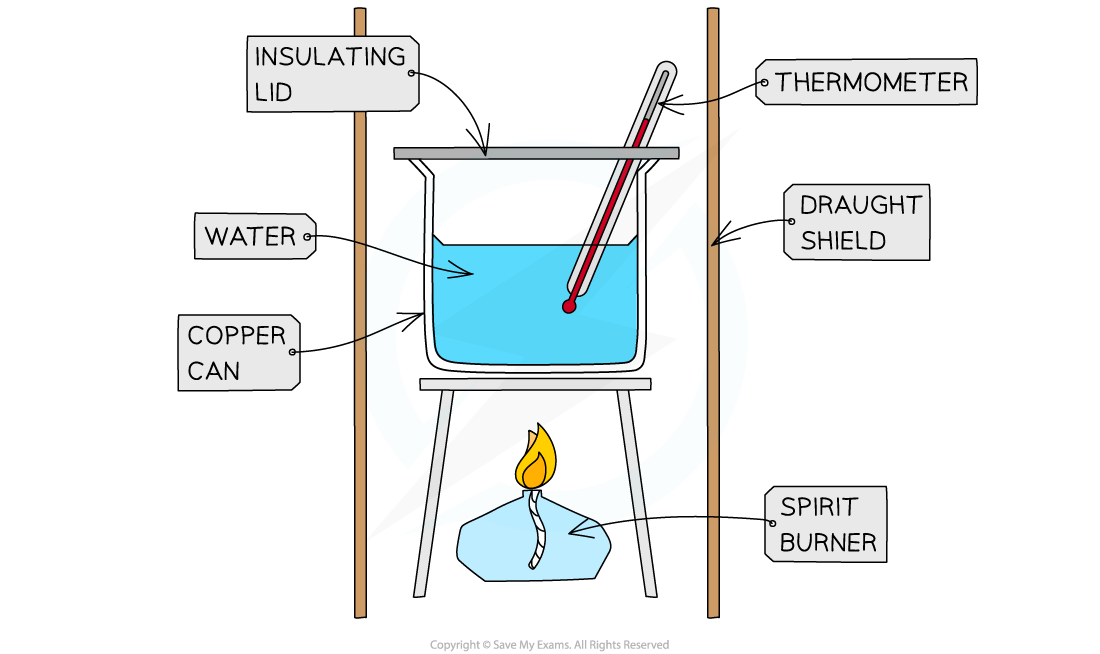
Two measurements are required for this experiment:
The mass of water being heated (m)
The temperature change of the water (ΔT)
These measurements, along with the specific heat capacity of water (c), are used in the Eh = cmΔT formula to calculate the heat energy released
Calorimetry calculations
To calculate the quantity of heat energy released, we use the formula given on page 3 of the SQA Data Booklet
The heat energy formula
Eh = cmΔT
Values in the equation:
Eh = the heat energy absorbed by the water (in kilojoules, kJ)
c = the specific heat capacity of the substance being heated
For water, this value is 4.18 (found in the Data Booklet)
m = The mass of the substance being heated (in kilograms, kg)
ΔT = The change in temperature (in degrees Celsius, °C)
ΔT = T(final) - T(initial)
How to use the heat energy formula
List all the variables (Eh, c, m, ΔT), identifying the one you need to find
Convert all masses to kilograms (kg) by dividing by 1000
Calculate the temperature change, if required
Substitute the known values into the formula
Rearrange the formula for the unknown value
Solve the formula for the unknown value
Examiner Tips and Tricks
This calculation is a very common source of errors in exams. You must use the correct units:
The mass of water (m) must be in kilograms (kg)
To convert grams to kg, divide by 1000
The final answer for Eh will be in kilojoules (kJ)
Worked Example
Calculating Eh:
When a fuel was burned, it was used to heat 200g of water. The temperature of the water rose from 20°C to 50°C. Calculate the heat energy absorbed by the water.
[2]
Answer:
List the variables:
Eh = ?
c = 4.18
m = 200 g = 0.2 kg
ΔT = 50 - 20 = 30 °C
Substitute the known values into the formula:
Eh = cmΔT
Eh = 4.18 x 0.2 x 30 [1 mark]
There is no rearrangement required for this calculation
Solve the formula for the unknown value:
Eh = 25.08 kJ [1 mark]
Worked Example
Calculating temperature change (ΔT):
50.16 kJ of heat energy was transferred to 1.2 kg of water. Calculate the temperature change of the water.
[2]
Answer:
List the variables:
Eh = 50.16
c = 4.18
m = 1.2 kg
ΔT = ?
Substitute the known values into the formula:
Eh = cmΔT
50.16 = 4.18 x 1.2 x ΔT [1 mark]
Rearrange the formula:
ΔT =
Solve the formula for the unknown value:
ΔT = 10 oC [1 mark]

Unlock more, it's free!
Was this revision note helpful?
