Born-Haber Cycle Calculations (HL) (DP IB Chemistry): Revision Note
Born-Haber cycle calculations
Once a Born–Haber cycle is set up, you can use Hess’s Law to calculate the lattice enthalpy (ΔHꝋlatt) by rearranging the following expression:
ΔHꝋf = ΔHꝋat + ΔHꝋat + ΔHꝋie + ΔHꝋea - ΔHꝋlatt
If we simplify this into three terms, this makes the equation easier to see:
ΔHꝋlatt
ΔHꝋf
ΔHꝋ1 (the sum of all the various enthalpy changes necessary to convert the elements in their standard states to gaseous ions)
The simplified equation becomes:
ΔHꝋf = ΔHꝋ1 - ΔHꝋlatt
Rearranging to calculate lattice enthalpy gives:
ΔHꝋlatt = - ΔHꝋf + ΔHꝋ1
A Born-Haber cycle could be used to calculate any stage in the cycle
You might be given ΔHꝋlatt and asked to work out ΔHꝋf
The process remains the same; apply Hess’s Law
Identify the direct and indirect routes in the cycle
Write an equation linking all relevant enthalpy changes, then rearrange to isolate the unknown value
Always consider the stoichiometry of the ions involved:
If you are dealing with ions like MgCl2, remember that values (e.g., for electron affinity) may need to be multiplied to reflect the number of moles involved
For example:
MgCl2 forms 2 Cl⁻ ions, so the electron affinity of chlorine must be doubled
You’re effectively adding 2 moles of electrons to 2 moles of chlorine atoms, forming 2 moles of Cl⁻
Examiner Tips and Tricks
You may need to refer to the Data Booklet for specific values, so take your time and ensure you select the correct data for each step in the cycle
Worked Example
Calculating the lattice enthalpy of KCl
Given the data below, calculate the ΔHꝋlatt of potassium chloride (KCl).
| ΔHꝋat (kJ mol-1) | ΔHꝋie / ΔHꝋea (kJ mol-1) |
|---|---|---|
K | +90 | +418 |
Cl | +122 | -349 |
ΔHꝋf (kJmol-1) | ||
KCl | -437 | |
Answer:
Step 1: Construct the Born-Haber cycle
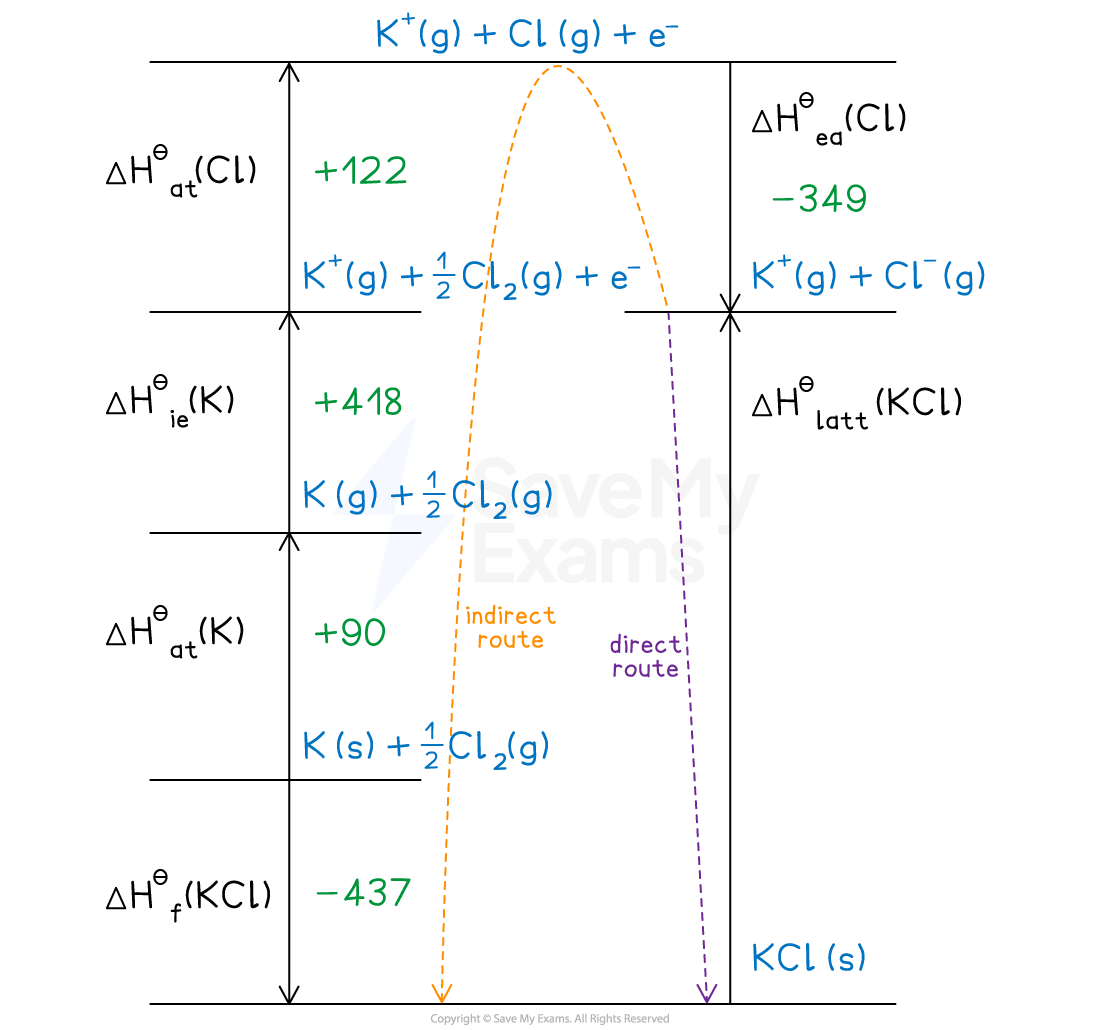
Step 2: Applying Hess’ law, the lattice enthalpy of KCl is:
ΔHꝋlatt = -ΔHꝋf + ΔHꝋ1
ΔHꝋlatt = -ΔHꝋf + [(ΔHꝋat K) + (ΔHꝋat Cl) + (ΔHꝋie K) + (ΔHꝋea Cl)]
Step 3: Substitute in the numbers:
ΔHꝋlatt = -(-437) + [(+90) + (+122) + (+418) + (-349)]
ΔHꝋlatt = 718 kJ mol-1
Worked Example
Calculating the lattice enthalpy of MgO
Given the data below, calculate the of ΔHꝋlat magnesium oxide of magnesium oxide (MgO)
| ΔHꝋat (kJ mol-1) | ΔHꝋie1 / ΔHꝋea1 (kJ mol-1) | ΔHꝋie2 / ΔHꝋea2 (kJ mol-1) |
|---|---|---|---|
Mg | +148 | +736 | +1450 |
O | +248 | -142 | +770 |
ΔHꝋf (kJmol-1) | |||
MgO | -602 | ||
Answer:
Step 1: Construct the Born-Haber cycle

Step 2: Applying Hess’ law, the lattice enthalpy of MgO is:
ΔHꝋlatt = -ΔHꝋf + ΔHꝋ1
ΔHꝋlatt = -ΔHꝋf + [(ΔHꝋat Mg) + (ΔHꝋat O) + (ΔHꝋie1 Mg) + (ΔHꝋie2 Mg) + (ΔHꝋea1 O) + (ΔHꝋea2 O)]
Step 3: Substitute in the numbers:
ΔHꝋlatt = -(-602) + [(+148) + (+248) + (+736) + (+1450) + (-142) + (+770)]
ΔHꝋlatt = 3812 kJ mol-1

Unlock more, it's free!
Was this revision note helpful?
