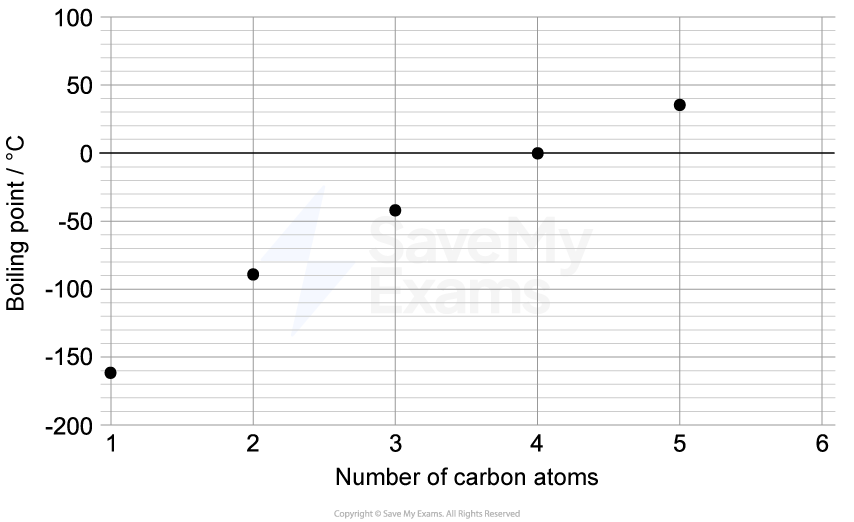
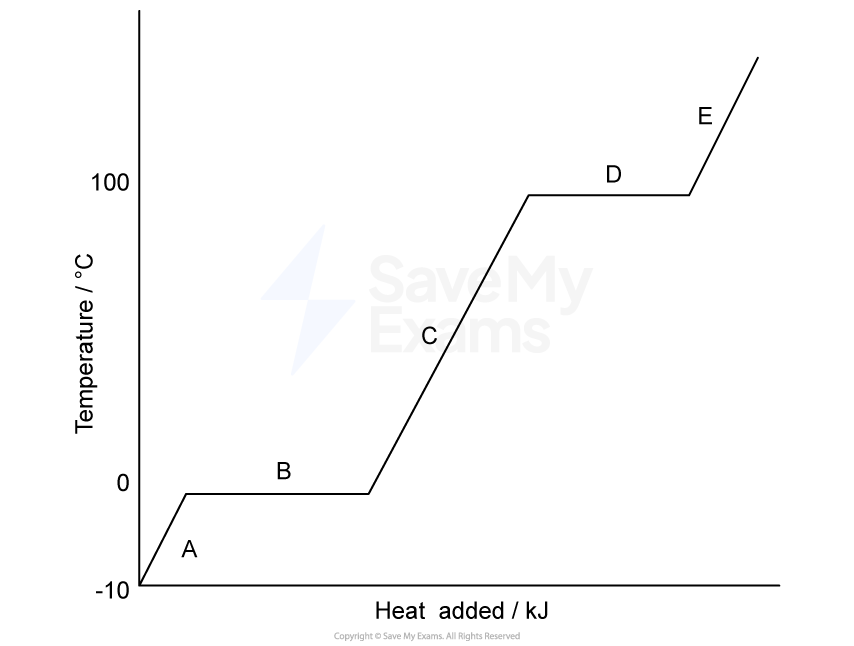
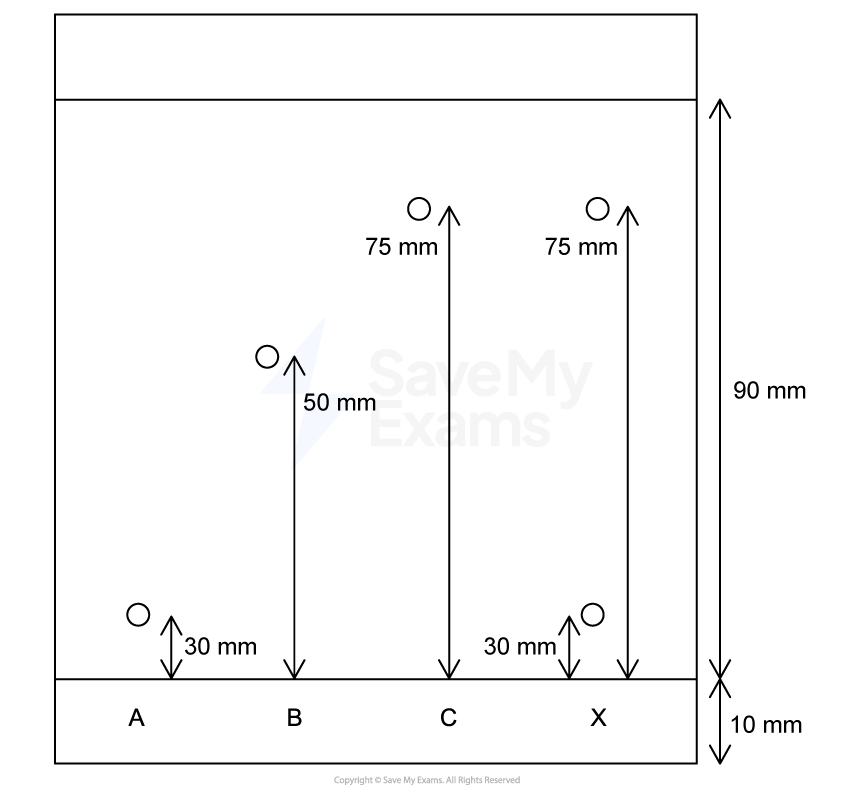
The properties of elements show predictable patterns across a period. The table below shows the atomic radius and first ionization energy for the elements in Period 3 of the periodic table.
Element | Atomic number | First ionisation energy / kJ mol-1 | Atomic radius / pm |
|---|---|---|---|
Sodium (Na) | 11 | 496 | 160 |
Magnesium (Mg) | 12 | 738 | 140 |
Aluminium (Al) | 13 | 578 | 124 |
Silicon (Si) | 14 | 787 | 114 |
Phosphorus (P) | 15 | 1012 | 109 |
Sulfur (S) | 16 | 1000 | 104 |
Chlorine (Cl) | 17 | 1251 | 100 |
Argon (Ar) | 18 | 1520 | 101 |
State the element in Period 3 that requires the least energy to remove one mole of its valence electrons.
Describe the general trend for:
i) Atomic radius from sodium to chlorine.
[1]
ii) First ionization energy from sodium to argon.
[1]
Explain the general increase in the first ionisation energy across Period 3.
Explain why the first ionisation energy of aluminium is lower than that of magnesium.
Explain how the data for first ionisation energy supports the fact that metallic character decreases across Period 3.
Was this exam question helpful?