The Gas Laws (SQA National 5 Physics): Revision Note
Exam code: X857 75
The gas laws
The gas laws are a set of three laws that describe the relationships between the temperature, pressure and volume of gases
Pressure & volume
Pressure is inversely proportional to volume
Where:
p = pressure in pascals (Pa)
V = volume in metres cubed (m3)
This means that:
The pressure-volume relationship only works when:
the gas is at a constant temperature
Therefore, a constant mean kinetic energy
the mass of the gas does not change
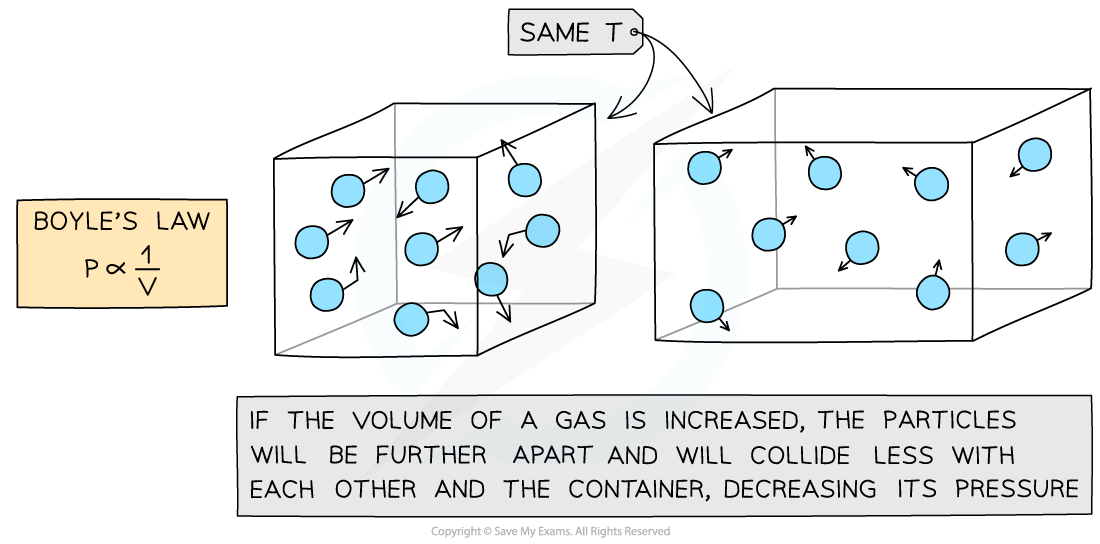
When the volume of the container is increased, the pressure decreases
The gas is expanded
The space in between the particles increases
The particles collide with the surfaces of the container less frequently
Resulting in a decrease in pressure
When the volume of the container is decreased, the pressure increases
The gas is compressed
The space in between the particles decreases
The particles collide with the surfaces of the container more frequently
Resulting in an increase in pressure
The
relationship can also be written as:
Where:
p1 = initial pressure in pascals (Pa)
V1 = initial volume in metres cubed (m3)
p2 = final pressure in pascals (Pa)
V2 = final volume in metres cubed (m3)
This relationship is often referred to as Boyle's Law
It is used to compare the pressure and volume before and after a change
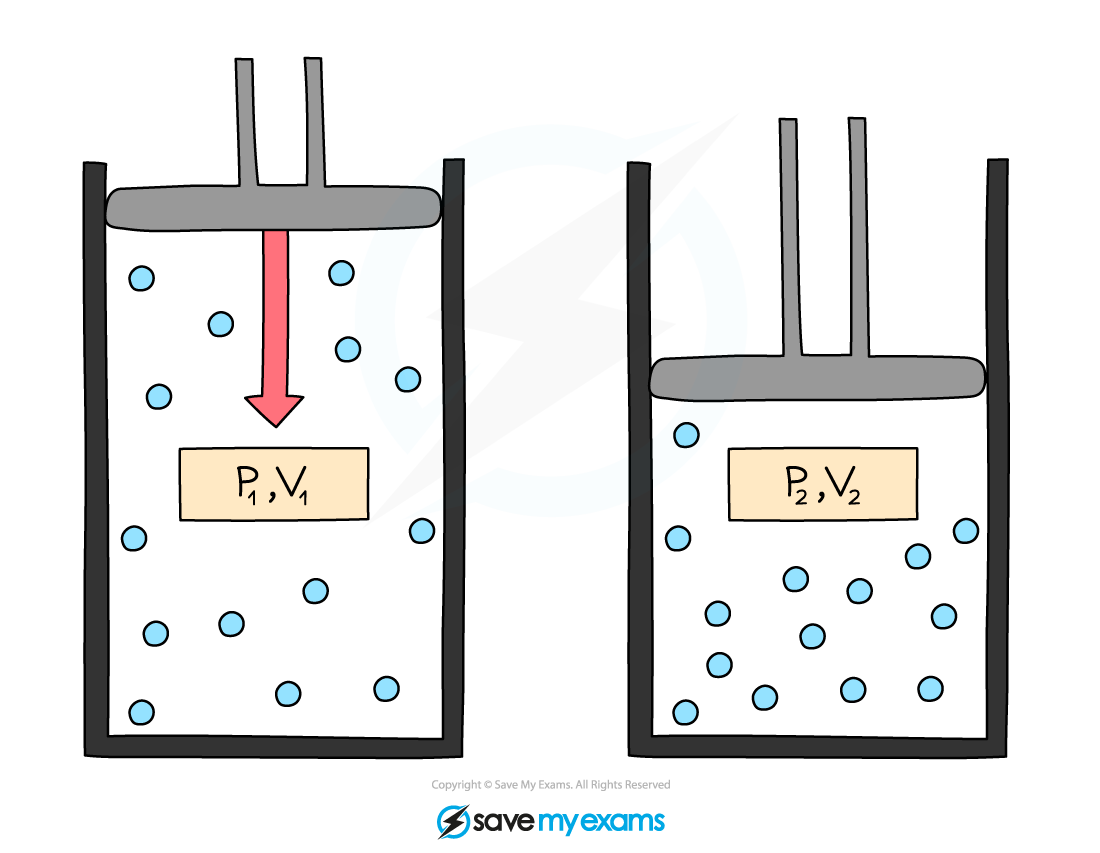
Worked Example
A gas occupies a volume of 0.70 m3 at a pressure of 203 Pa.
Calculate the pressure exerted by the gas after it has been compressed to a volume of 0.15 m3.
Assume that the temperature and mass of the gas stay the same.
Answer:
Step 1: List the known variables
Initial pressure, p1 = 203 Pa
Initial volume, V1 = 0.70 m3
Final volume, V2 = 0.15 m3
Step 2: Write out the relationship
Step 3: Rearrange the relationship to make p2 the subject
Divide both sides by V2
Step 4: Substitute in the known values to calculate
Step 5: Round to an appropriate number of significant figures
The least precise input value is 2 s.f.
Therefore, the answer can only be given to the same precision
Examiner Tips and Tricks
Always check whether your final answer makes sense.
If the gas has been compressed, the final pressure is expected to be more than the initial pressure. If this is not the case, double-check the rearranging of any relationships and the values put into your calculator.
One pascal is a very small amount of pressure, and you will typically meet pressures in the order of kilo-pascals.
The pressure exerted on you right now due to air pressure is equal to 100 kPa, so use this as a reference when considering if your answer makes sense.
Pressure & temperature
Pressure is directly proportional to temperature
Where:
p = pressure in pascals (Pa)
T = temperature in Kelvin (K)
This means that:
The pressure-temperature relationship only works when:
the gas is at a constant volume
the mass of the gas does not change
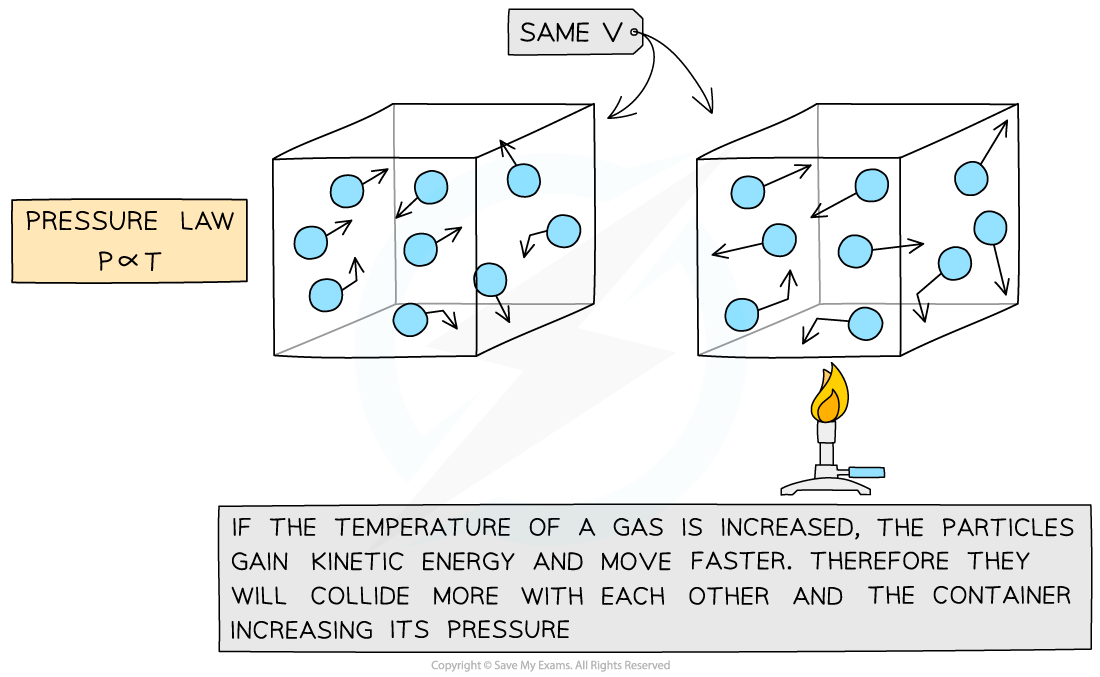
When the temperature of the gas is increased, the pressure increases
The mean kinetic energy of the particles is increased
The particles travel faster
The particles collide with the surfaces of the container more frequently
The force of each collision increases
Resulting in an increase in pressure
When the temperature of the gas is decreased, the pressure decreases
The mean kinetic energy of the particles is decreased
The particles travel more slowly
The particles collide with the surfaces of the container less frequently
The force of each collision decreases
Resulting in a decrease in pressure
This relationship can also be written as:
Where:
P1 = initial pressure (Pa)
P2 = final pressure (Pa)
T1 = initial temperature (K)
T2 = final temperature (K)
This relationship is sometimes referred to as the pressure law or Gay-Lussac's law
It can be used to compare the pressure and temperature before and after a change
Worked Example
The pressure inside a bicycle tyre is 5.10 × 105 Pa when the temperature is 279 K. After the bicycle has been ridden, the temperature of the air in the tyre is 299 K.
Calculate the new pressure in the tyre, assuming the volume is unchanged.
Answer:
Step 1: List the known quantities
Initial pressure,
Initial temperature,
Final temperature,
Step 2: Write out the relevant relationship
Step 3: Rearrange to make the subject
Multiply both sides by
Step 4: Substitute in the known values to calculate
Step 5: Round to an appropriate amount of significant figures
The least precise input value is 3 s.f.
Therefore, the answer can only be given to this level of precision
Volume & temperature
Volume is directly proportional to temperature
Where:
V = volume in metres cubed (m3)
T = temperature in Kelvin (K)
This means that:
The volume-temperature relationship only works when:
the gas is at a constant pressure
the mass of the gas does not change
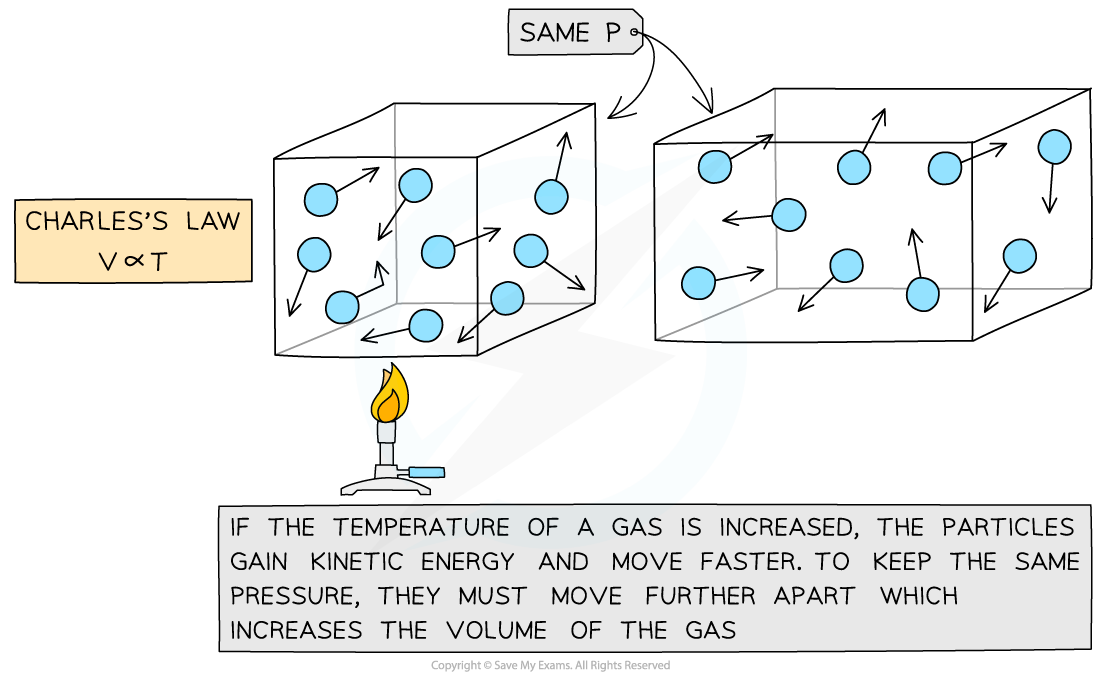
When the temperature of the gas is increased, the volume increases
The mean kinetic energy of the particles is increased
The particles travel faster
The space in between the particles increases
Resulting in an increase in volume
When the temperature of the gas is decreased, the volume decreases
The mean kinetic energy of the particles is decreased
The particles travel more slowly
The space in between the particles decreases
Resulting in a decrease in volume
This relationship can also be written as:
Where:
V1 = initial volume (m3)
V2 = final volume (m3)
T1 = initial temperature (K)
T2 = final temperature (K)
This relationship is often referred to as Charles' Law
It is used to compare the volume and temperature before and after a change
Examiner Tips and Tricks
Note that all temperature values must be in Kelvin for the gas law calculations. If temperatures are given in °C, then you must convert them to Kelvin. See the revision note Absolute Zero for a reminder on how to do this.

Unlock more, it's free!
Was this revision note helpful?
