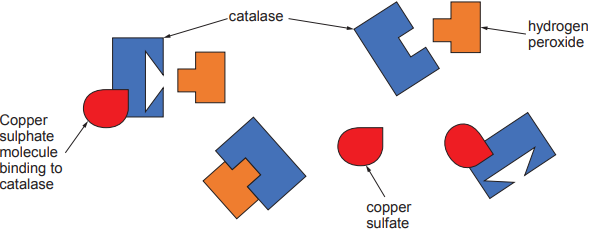
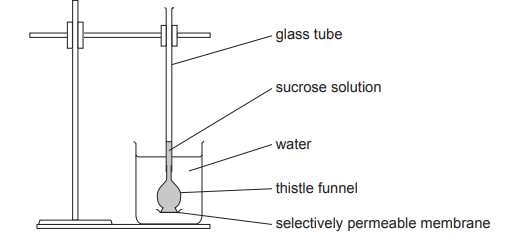
Protease and lipase are enzymes that are added to biological washing powders to help break down stains on clothing.
Complete the sentences below by stating the two types of molecules that the enzymes in the biological washing powders help to break down.
Protease breaks down ………………..................................…………………. .
Lipase breaks down ………………..................................…………………. .
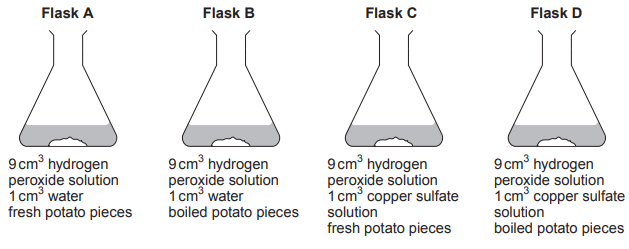
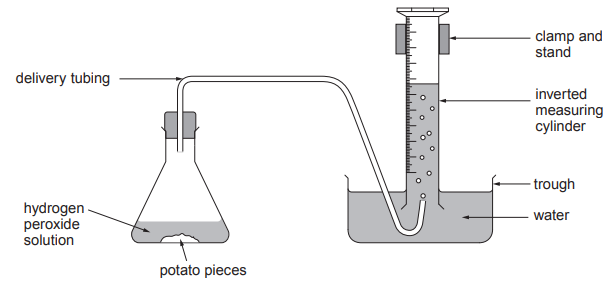
Students designed an experiment to test the effect of temperature on the activity of these enzymes in biological washing powder. They used the following method:
Stain five pieces of fabric with egg.
Set up five beakers of water at 20°C, 30°C, 40°C, 50°C and 60°C.
Add biological washing powder to each beaker.
Add a piece of stained fabric to each beaker and leave for 5 minutes.
Remove the samples of fabric and compare the stain remaining.
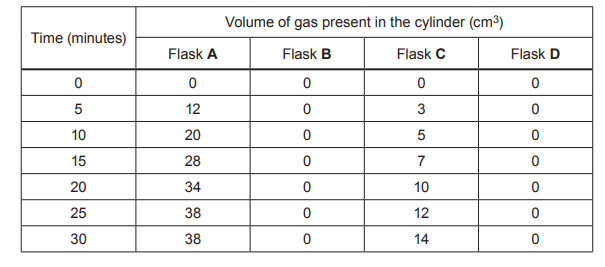
The method and results of the experiment are shown in Image 6.1.
Image 6.1
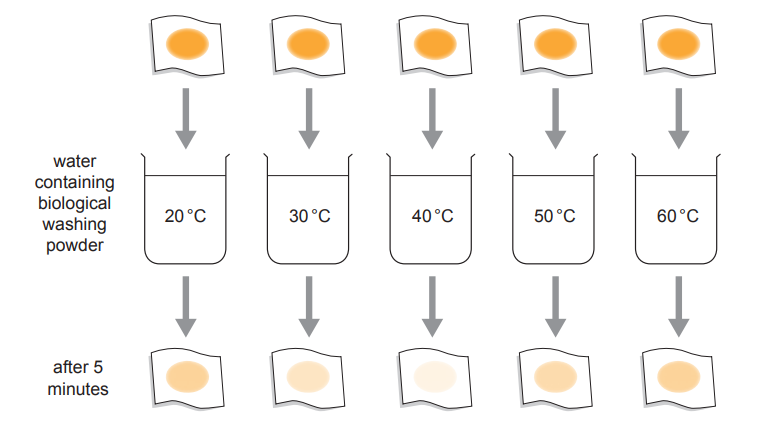
(i) Suggest the best temperature to wash clothes using this biological washing powder. Use the results in Image 6.1 to give the reason for your answer.
[2]
Temperature …………….................... °C Reason
(ii) Use your knowledge of enzymes to explain the result at 60°C.
[3]
The students repeated the experiment using the same method but using non-biological washing powder which does not contain enzymes. Their results are shown in Image 6.2.
Image 6.2
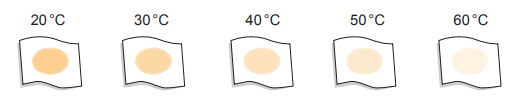
(i) Describe the results with the non-biological washing powder.
[1]
(ii) State why they repeated the experiment using non-biological washing powder.
[1]
(iii) Use the results of both experiments to explain the advantage to the environment of adding enzymes to washing powder.
[2]
Suggest two variables which should have been controlled in this method.
Was this exam question helpful?