In 2018–19, there was an outbreak of the Ebola virus disease in the Democratic Republic of the Congo (DRC). A double-blind clinical trial was carried out in four villages on 499 people infected with the Ebola virus. The purpose was to test the effectiveness of four potential treatments against the virus.
Table 9 shows the results halfway through the trial in August 2019.
Table 9
Treatment | Percentage of infected people dying from Ebola following the treatment (%) |
REGN-EB3 | 29 |
mAb-114 | 33 |
ZMapp | 49 |
Remdesivir | 53 |
At the time of the trial, 1900 people out of 2831 confirmed cases of Ebola in other parts of the DRC had died of the disease. None of the 2831 people were part of the trial and so did not receive any of the four treatments above.
(i) Use the information given above to calculate the percentage of confirmed cases of Ebola who received no treatment and died of the disease. Give your answer to two significant figures.
[2]
Percentage = ..........................
(ii) The results shown in Table 9 convinced the scientists to stop using ZMapp and Remdesivir, and place all remaining patients on either REGN-EB3 or mAb-114.
Suggest why the scientists stopped using ZMapp and Remdesivir in August 2019 and placed all the remaining patients on either REGN-EB3 or mAb-114.
[1]
(iii) Explain the meaning and importance of double-blind trials.
[2]
(iv) This trial did not use a placebo. Suggest why a placebo could be considered unethical in this trial.
[1]
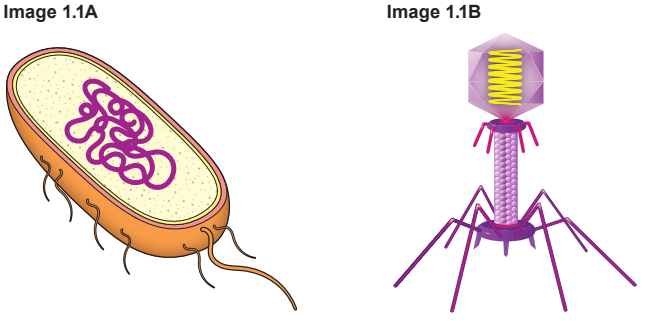
Complete Table 1.2 by placing a tick (✓) in the correct column for each statement.