Half-Life (SQA National 5 Physics): Revision Note
Exam code: X857 75
Half-life
Half-life is defined as:
The time taken for the activity of a radioactive source to halve
The activity is the number of nuclear disintegrations per unit time
Since radioactive decay is a random process, it is impossible to know when any particular unstable nucleus will decay
However, the decrease in activity can be measured and predicted
Activity decreases with time because the number of unstable nucleii gradually decreases
This leaves fewer and fewer unstable nuclei available to decay and emit radiation
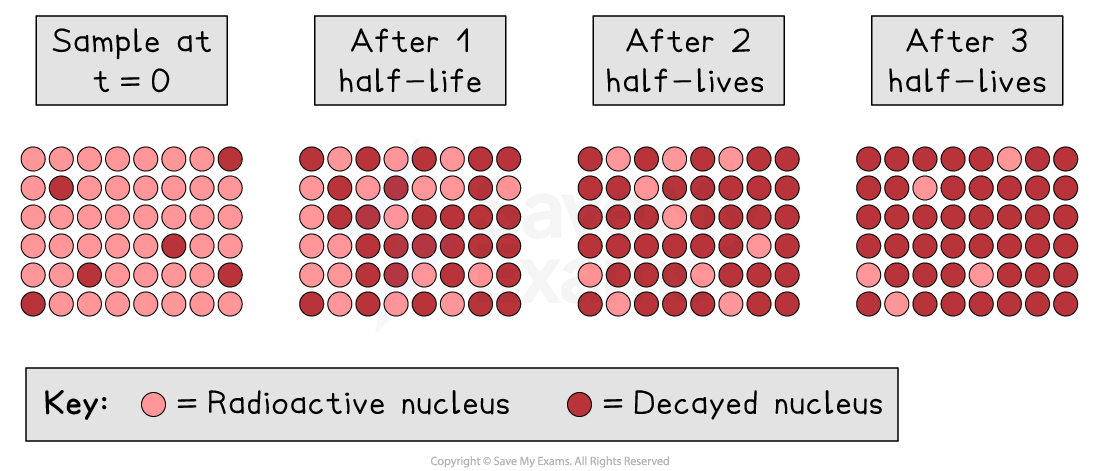
Different radioactive substances have different half-lives
Half-lives can vary from a fraction of a second to billions of years in length
Measuring half-life
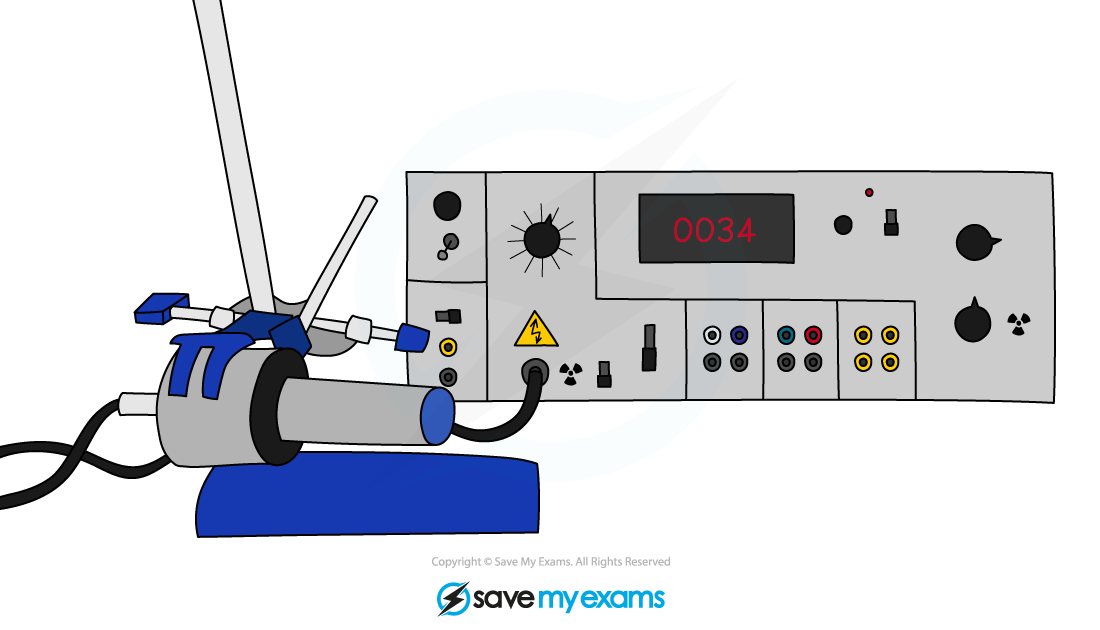
To determine the half-life of a sample, the procedure is:
Measure the initial activity A0 of the sample using a Geiger counter
Correct for back ground radiation
Determine the half-life of this original activity
Measure how the activity changes with time
The time taken for the activity to decrease to half its original value is the half-life
Accounting for background radiation
Background radiation must be accounted for when taking readings in a laboratory
This can be done by taking readings with no radioactive source present and then subtracting this from readings with the source present
This is known as the corrected count rate
Measuring background count rate
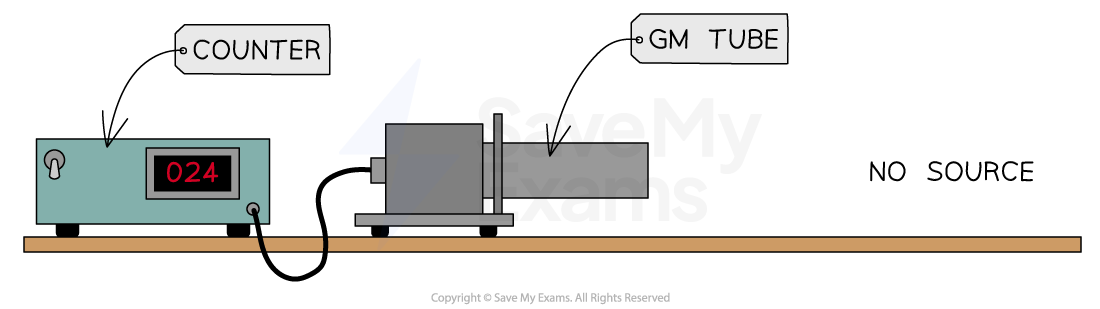
For example, if a Geiger counter records 24 counts in 1 minute when no source is present, the background radiation count rate would be:
24 counts per minute
24/60 = 0.4 counts per second
Measuring the corrected count rate of a source
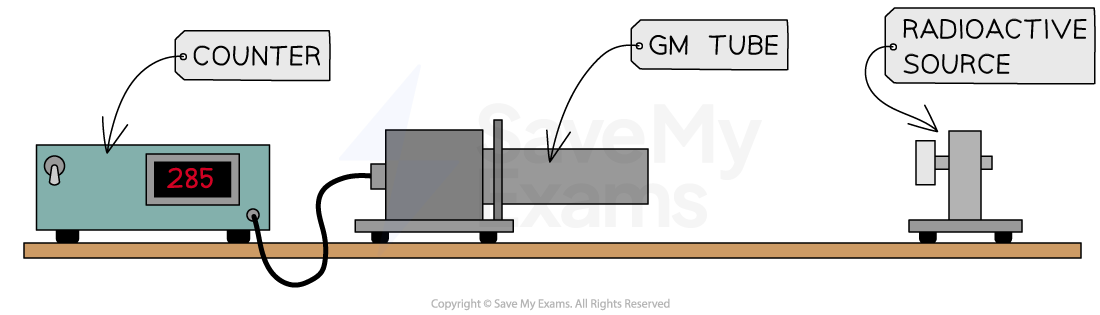
Then, if the Geiger counter records, for example, 285 counts in 1 minute when a source is present, the corrected count rate would be:
285 − 24 = 261 counts per minute
261/60 = 4.35 counts per second
When measuring count rates, the accuracy of results can be improved by:
Repeating readings and taking averages
Taking readings over a long period of time
Uses of half-life
The half-life of a radioactive substance makes it suitable for some purposes and unsuitable for others
For example:
The half-life of a medical tracer must be long enough to last through the procedure, but short enough to quickly leave the patient’s body and minimize harm
The half-life of the alpha source in a smoke alarm must be long enough to ensure steady emissions but not so short that it needs frequent replacement
Worked Example
State what is meant by the term half-life.
Answer:
Half-life is the time taken for the activity of a radioactive source to fall to half of its original value
Examiner Tips and Tricks
Examiners are very strict on the language you can use to define half-life, so it is best to learn this definition for your exam.
Other acceptable answers include:
The time taken for the activity to halve
The time taken for half the radioactive nuclei to decay/disintegrate
Answers that were not accepted:
Time for radiation to halve
Time for radioactivity to halve
Time for count rate to halve
Examiners are looking for you to be specific that it is the activity, or the number of radioactive nucleii that halves.
Calculating half-life
Scientists can measure the half-lives of different isotopes accurately
Uranium-235 has a half-life of 704 million years
This means it would take 704 million years for the activity of a uranium-235 sample to decrease to half its original amount
Carbon-14 has a half-life of 5700 years
So after 5700 years, there would be 50% of the original amount of carbon-14 remaining
After two half-lives or 11 400 years, there would be just 25% of the original amount of carbon-14 remaining
With each half-life, the amount of undecayed radioactive nuclei remaining decreases by half
A graph can be used to make half-life calculations
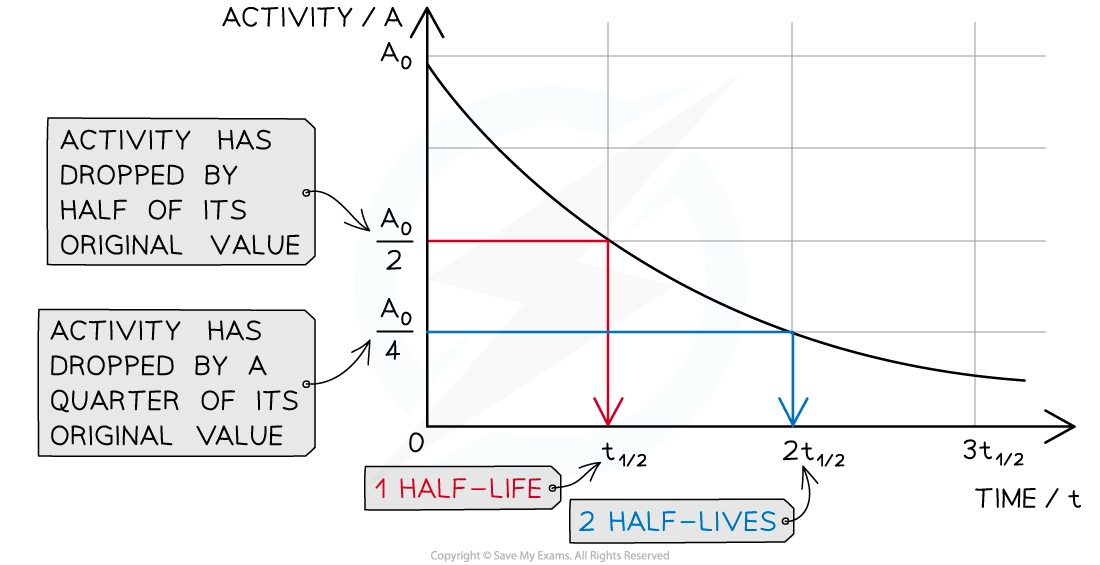
The time it takes for the activity of the sample to decrease from 100% to 50% is the half-life
It is the same length of time as it would take to decrease from 50% activity to 25% activity
The half-life is constant for a particular substance
The following table shows that as the number of half-life increases, the proportion of the isotope remaining halves
Half life calculation table
number of half lives | proportion of isotope remaining |
|---|---|
0 |
|
1 |
|
2 |
|
3 |
|
4 |
|
Worked Example
The activity of a particular radioactive sample is 880 Bq.
After one year, the activity has dropped to 220 Bq.
What is the half-life of this material?
Answer:
Step 1: Calculate how many times the activity has halved
Initially, the activity was 880 Bq
After 1 half-life the activity would be 440 Bq
After 2 half-lives, the activity would be 220 Bq
Therefore, 2 half-lives have passed
Step 2: Divide the time period by the number of half-lives
The time period is a year
The number of half-lives is 2
So two half-lives is 1 year, and one half-life is 6 months
Therefore, the half-life of the sample is 6 months
Worked Example
The activity of a radioactive sample of rhodium-106 is measured.
The graph shows how the activity of the sample varies with time.

Use the graph to determine the activity after three half-lives have passed.
Answer:
Step 1: Draw lines on the graph to determine the time it takes for the sample to drop to half of its original activity

This is the half-life of the sample
Step 2: Draw lines on the graph to determine the activity after another half-life

Step 3: Draw lines on the graph to determine the activity after another half-life

The activity after 3 half-lives is 800 Bq
Examiner Tips and Tricks
Determining half-life requires using either graphical data or numerical data. You could be presented with either in your exam, so make sure you are comfortable using both.

Unlock more, it's free!
Was this revision note helpful?
