Preparation of Soluble Salts From Metal Oxides or Carbonates (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Producing soluble salts from carbonates or oxides
When an acid reacts with an insoluble base (like a metal oxide or metal carbonate), a titration cannot be used
This is because the solid base would block the burette
Instead, a different method is used which takes advantage of the fact that the base is a solid we can see and easily remove
The "excess" method
The key to this method is adding more of the insoluble base than is needed to make sure the acid has been neutralised
Why add in excess?
If the acid is not all used up, the final salt will be impure, contaminated with leftover acid
By adding excess base, it is guaranteed that the acid is the limiting reactant and is completely used up
The leftover base is then easily removed by filtration
How do we know it's in excess?
Unreacted solid base left is seen at the bottom of the beaker
It will have stopped reacting (e.g., the fizzing stops for a carbonate)
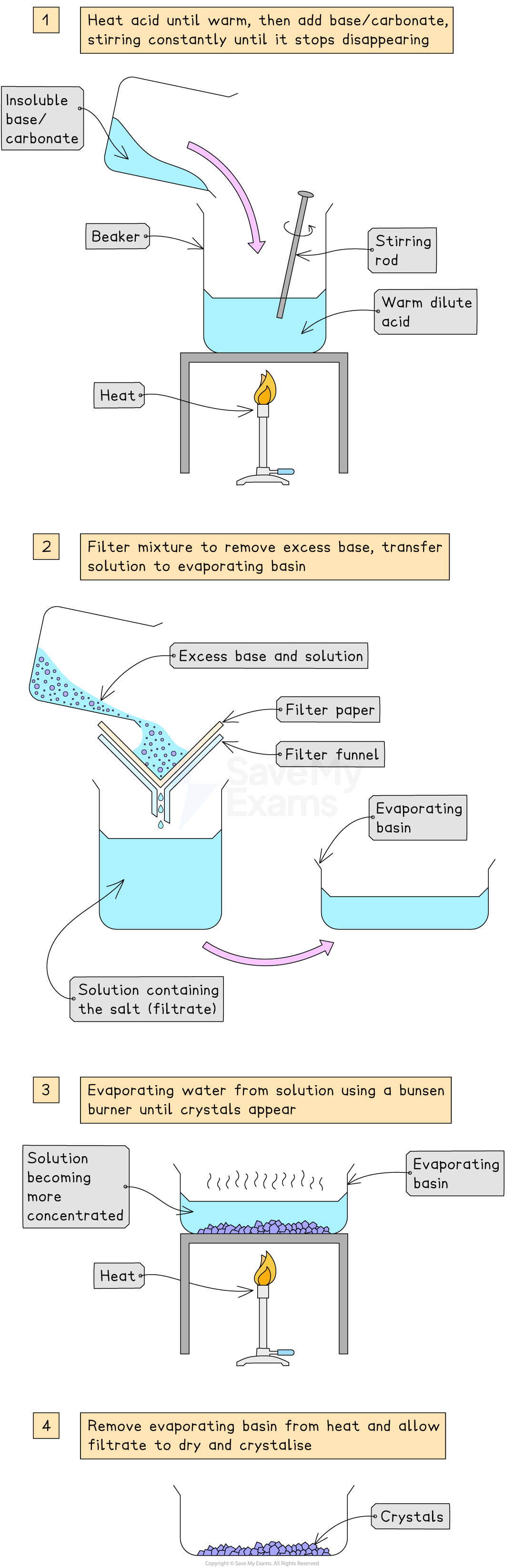
Method
Warm dilute acid gently in a beaker
Add the insoluble base, or carbonate slowly while stirring until no more reacts
This means that the base is in excess
Filter the mixture to remove the excess solid
Transfer the filtrate (salt solution) to an evaporating basin and heat gently until the solution is concentrated
Check the solution is saturated by dipping a cold, glass rod into the solution and seeing if crystals form on the end
Leave the basin in a warm place to crystallise
Decant excess liquid and dry the crystals with filter paper
For example, preparing pure, hydrated copper(II) sulfate crystals using this method:
copper(II) oxide + sulfuric acid → copper(II) sulphate + water
CuO (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l)
Examiner Tips and Tricks
You must know the purpose of each key step:
"Why was the base added in excess?"
To ensure all of the acid was neutralised / reacted
"Why was the mixture filtered?"
To remove the unreacted / excess solid base
"What is the name of the clear liquid collected after filtering?"
The filtrate
Worked Example
A student wants to prepare a pure sample of the salt zinc chloride using an insoluble base.
a) Name the acid the student should use.
[1]
b) Name a suitable insoluble base the student could use.
[1]
Answer:
The name of the salt gives you all the clues you need
The first part of the name (zinc) tells you the metal that must be in the base
The second part of the name (chloride) tells you which acid to use:
Chloride salts are made from hydrochloric acid
Sulfate salts are made from sulfuric acid
Nitrate salts are made from nitric acid
a) Hydrochloric acid [1 mark]
b) Zinc oxide, zinc carbonate or zinc hydroxide [1 mark]
Page 8 of the data booklet gives solubilities of selected compounds in water

Unlock more, it's free!
Was this revision note helpful?
