Atomic Structure (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Structure of the atom
All substances are made of tiny particles of matter called atoms which are the building blocks of all matter
The atom is the smallest part of an element that still retains the properties of that element
However, atoms themselves are made of even smaller particles called subatomic particles
The three subatomic particles are:
Protons
Neutrons
Electrons
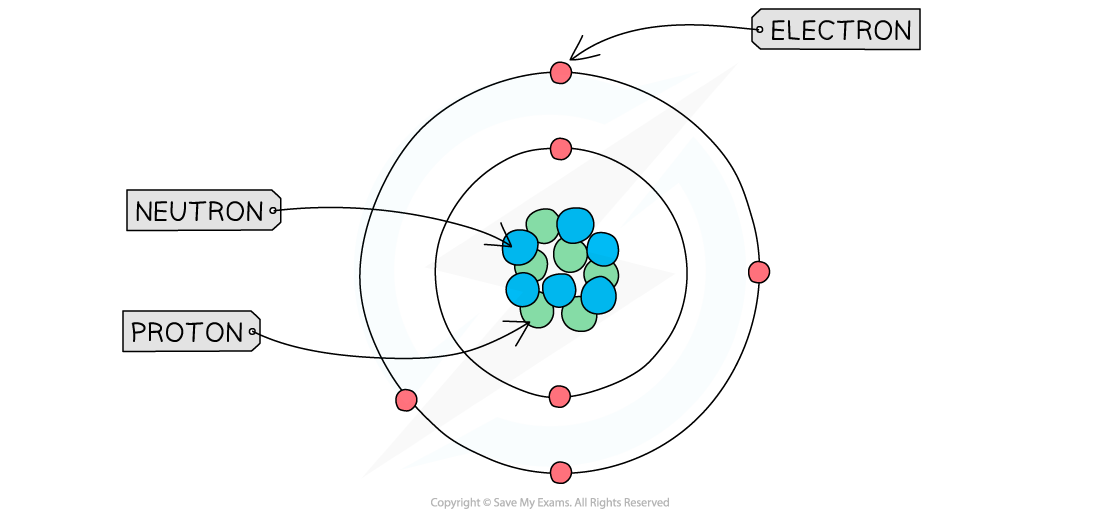
Location of subatomic particles
The particles are found in two main areas of the atom:
The nucleus:
This is the dense core at the centre of the atom
It contains the protons and neutrons
The nucleus is positively charged
Electron shells:
These are energy levels that orbit the nucleus
They contain the electrons, which move very fast
Atomic structure diagram
Relative mass & charge
The actual mass and charge of the subatomic particles are incredibly small
To make them easier to work with, we use a simplified, relative scale
The relative mass of subatomic particles
Particle | Relative mass |
|---|---|
Proton | 1 |
Neutron | 1 |
Electron | 1/2000 (almost 0) |
Since the mass of an electron is so small, the mass of an atom is almost entirely contained in the nucleus
The relative charge of subatomic particles
Particle | Charge |
|---|---|
Proton | +1 |
Neutron | 0 (neutral) |
Electron | -1 |
Overall, atoms are electrically neutral
This is because the negative charge of an electron exactly cancels out the positive charge of a proton
An ion is formed when an atom loses or gains electrons to achieve a full outer shell
If an atom loses electrons:
It has more positive protons than negative electrons
So, it forms a positively charged ion
If an atom gains electrons:
It has more negative electrons than positive protons
So, it forms a negatively charged ion
Worked Example
Explain why a magnesium ion has a 2+ charge.
[2]
Answer:
A magnesium atom has:
12 positively charged protons
12 negatively charged electrons
[1 mark]
To gain a full outer shell, magnesium loses two electrons
It now has 12 protons but only 10 electrons
So, the overall charge is 2+
[1 mark]
Examiner Tips and Tricks
In an exam, the mass of an electron can be stated as 'almost zero' or 'negligible'

Unlock more, it's free!
Was this revision note helpful?
