Common Chemical Apparatus (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Chemical apparatus
In chemistry, using the right tool for the job is essential for getting accurate and reliable results
For this course, you should be familiar with the names and main uses of the following common pieces of laboratory apparatus
General apparatus
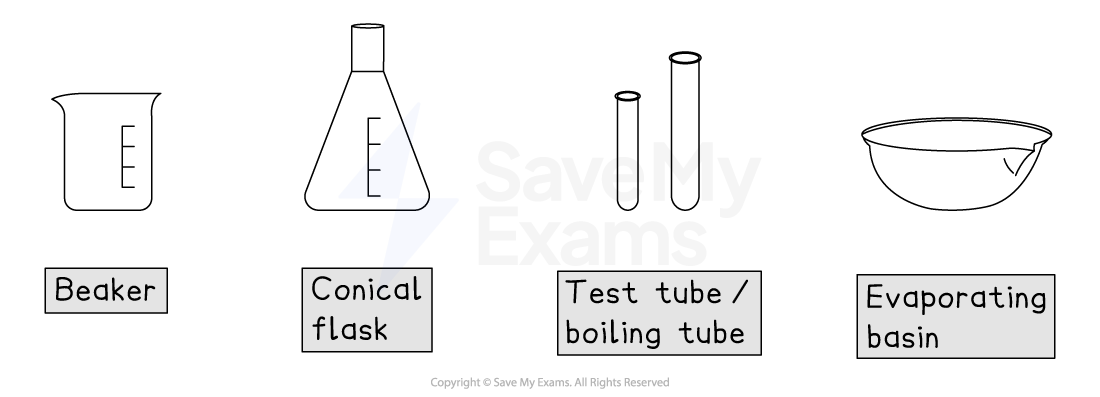
Beaker
This is a glass (or plastic) container used for holding, mixing and heating liquids
The scale on the side is for approximate volumes only
Conical flask
This flask has a narrow neck to prevent splashing and a wide base
This makes it ideal for swirling liquids to ensure they mix well during a reaction like a titration
Test tube / boiling tube
Test tubes and boiling tubes are used for observing chemical changes or reactions on a small scale
Boiling tubes are wider and made of tougher glass to allow for stronger heating
Evaporating basin
This is a shallow, ceramic dish used for evaporating a solvent from a solution, often to leave behind solid crystals.
Apparatus for measuring
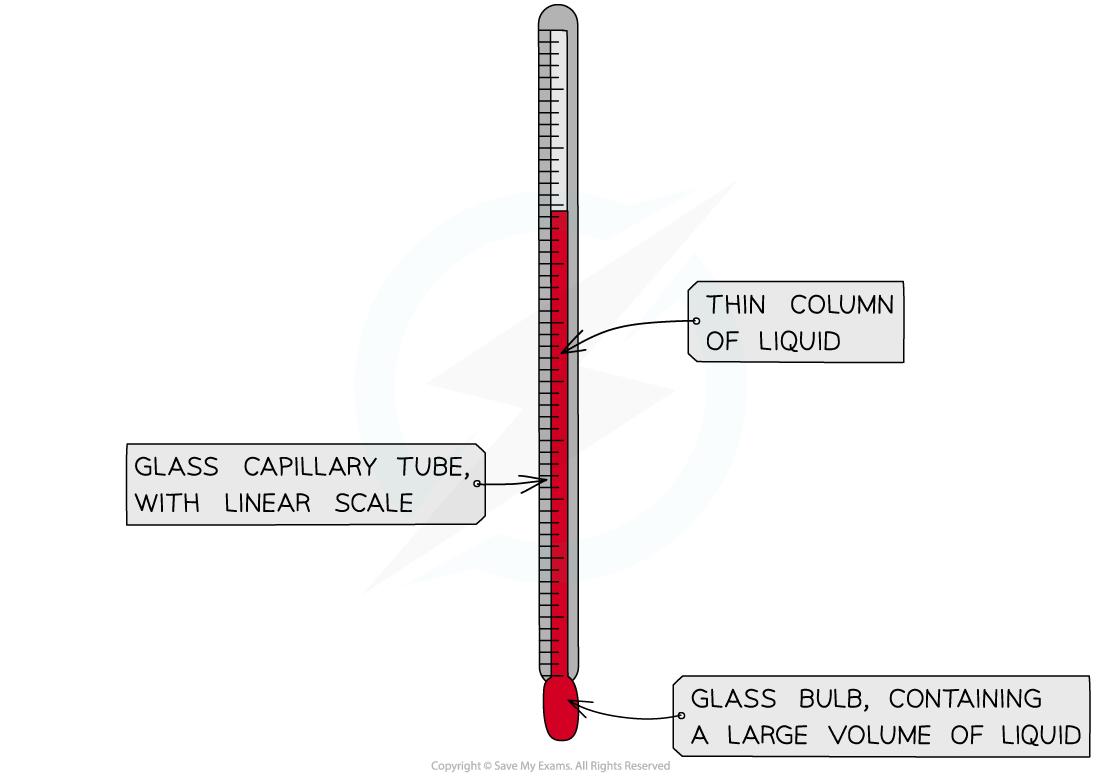
Thermometer
A thermometer is used to measure temperature in degrees Celsius (°C)
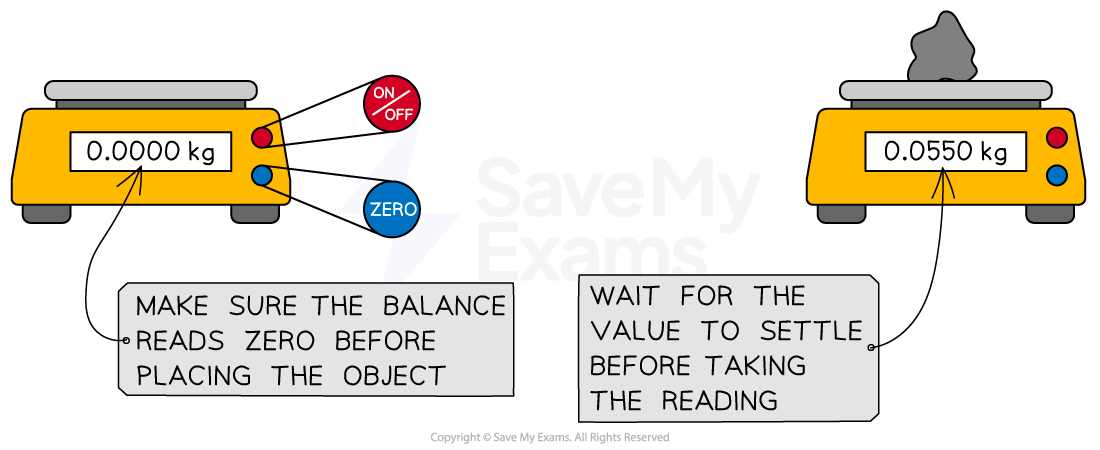
Digital balance
A digital balance is used to measure mass accurately
The standard unit of mass in kilograms (kg)
However, in chemistry grams (g) are most often used
Examiner Tips and Tricks
Always remember to press the tare (or zero) button before adding your substance to a digital balance
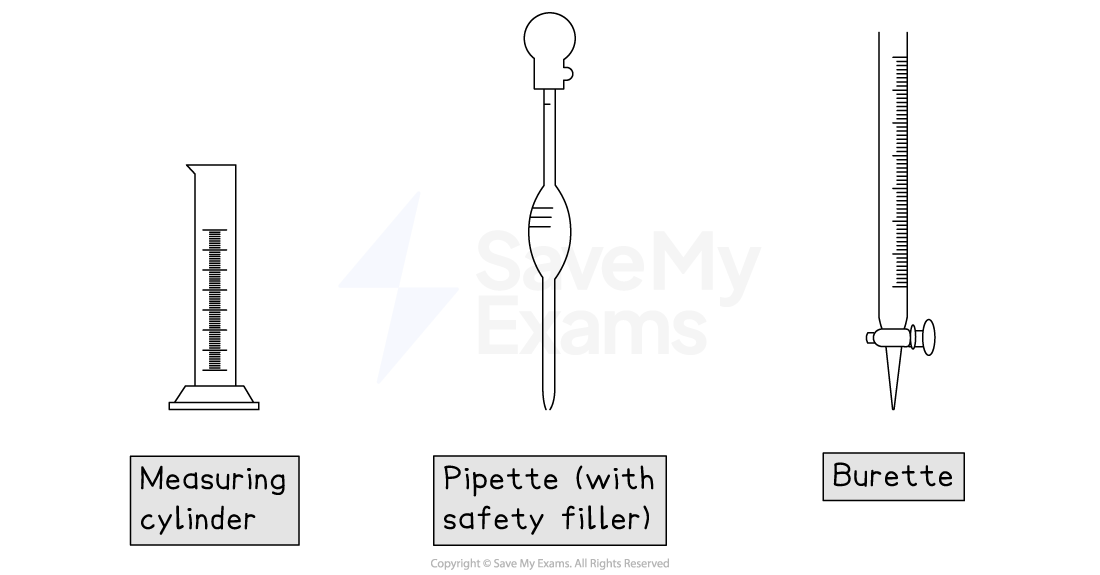
Measuring cylinder
A measuring cylinder is used for approximately measuring a range of volumes
Measurements are read from the bottom of the meniscus
Pipette (with safety filler)
Pipettes are used to accurately measure and transfer a single, fixed volume of liquid (e.g., exactly 10.0 cm3 or 25.0 cm3)
The safety filler is used to draw liquid into the pipette
There are various versions of safety fillers
Burette
A burette is a long, graduated tube with a tap at the bottom
It is used to add a variable but highly accurate volumes of liquid
It is essential for titrations, allowing you to measure the volume added precisely
Important:
The scale on a burette is read from top to bottom
So, 0.00 cm3 is at the top of the burette
Examiner Tips and Tricks
A common exam question is to choose the best piece of apparatus for measuring a volume
For approximate volumes: measuring cylinder
For an accurate fixed volume: pipette
For an accurate variable volume: burette
Other essential apparatus
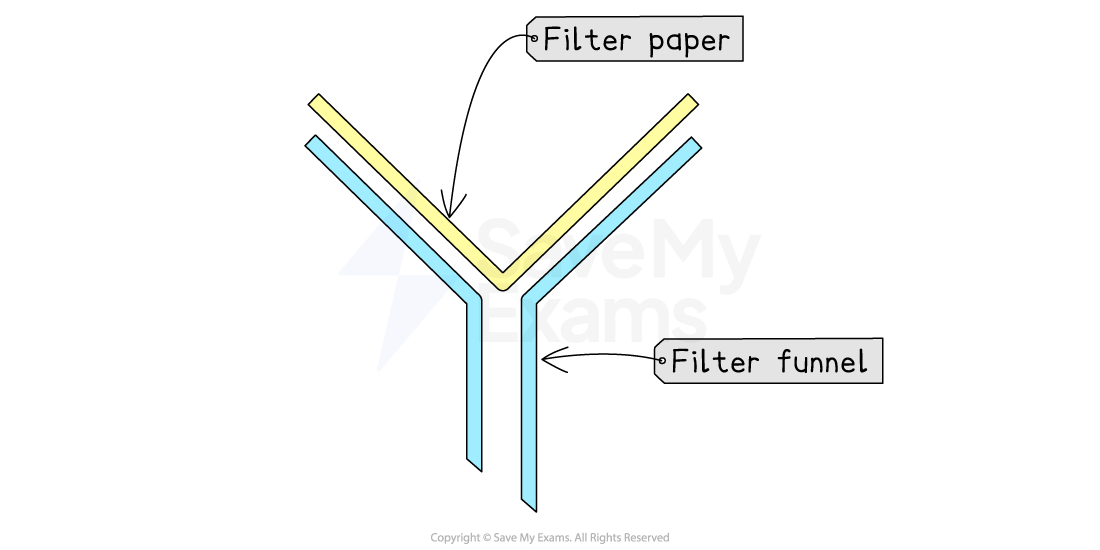
Funnel & filter paper
A funnel and filter paper is used to separate an insoluble solid from a liquid
e.g., sand from water
This process is called filtration
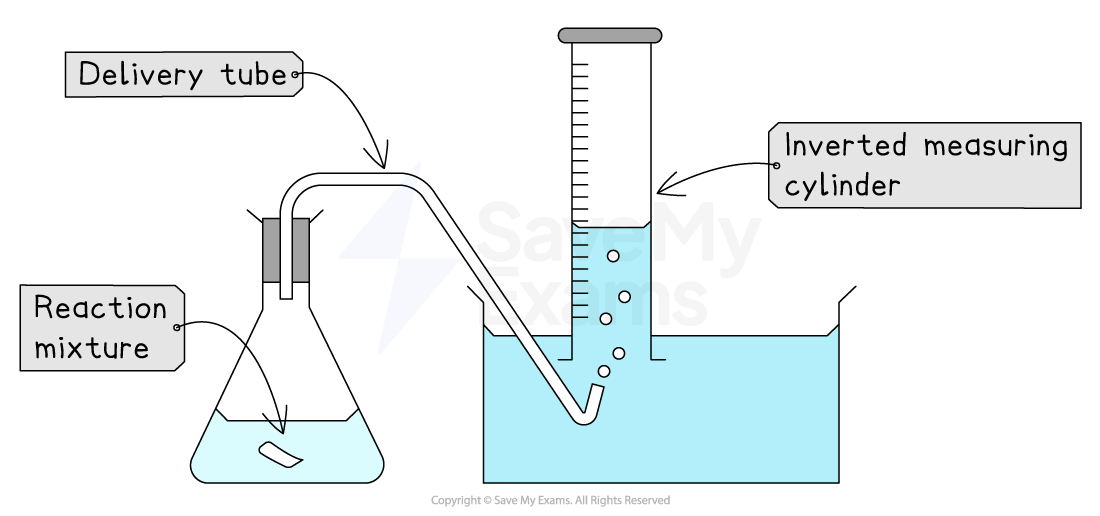
Delivery tube
This is a bent tube used to transfer a gas from a reaction vessel into a collection apparatus
This is most commonly used when collecting gases
The shape of the delivery tube depends on its specific job
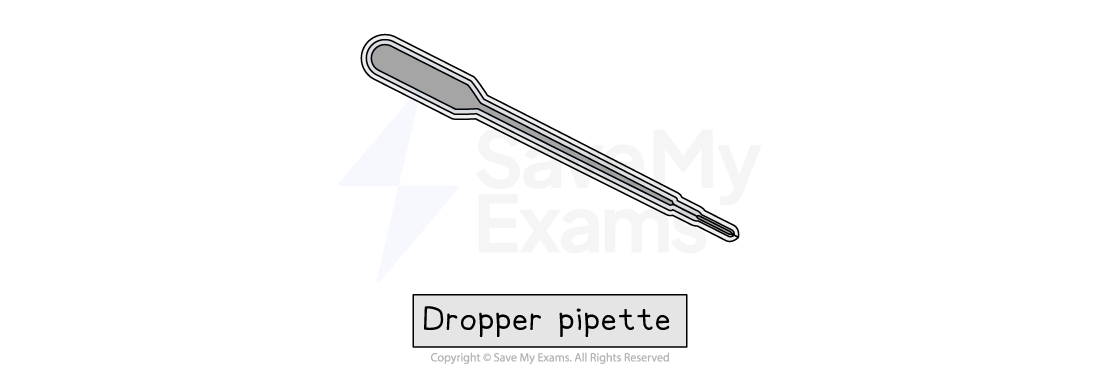
Dropper (or teat pipette)
Dropper or teat pipettes are used for adding a liquid one drop at a time
They are useful when only a very small amount is needed

Unlock more, it's free!
Was this revision note helpful?
