General Practical Techniques (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
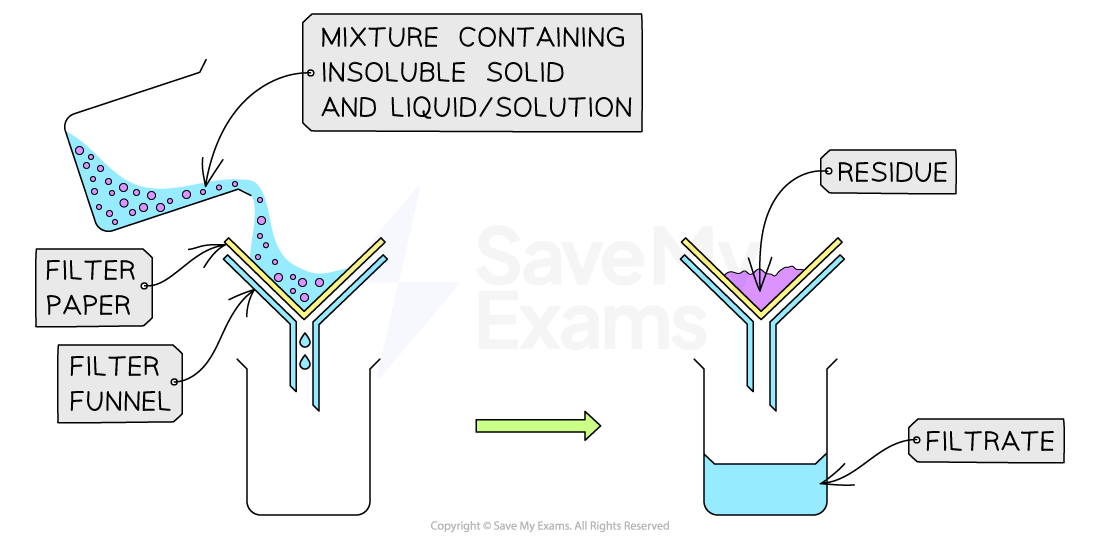
Simple filtration
Filtration is a technique used to separate an insoluble solid from a liquid
It is commonly used to:
Remove insoluble impurities
Collect insoluble products
Examples of when filtration can be used include:
Removing sand from a dissolved mixture of rock salt
Collecting a solid product from a precipitation reaction
How to filter
Fold a piece of filter paper and place it inside a funnel
Place the funnel over a conical flask or beaker
Pour the mixture through the filter paper
The liquid that passes through the filter paper is called the filtrate
The filtrate is a solution
The solution is made of the liquid / solvent and any dissolved substances
The solid that is trapped in the filter paper is called the residue
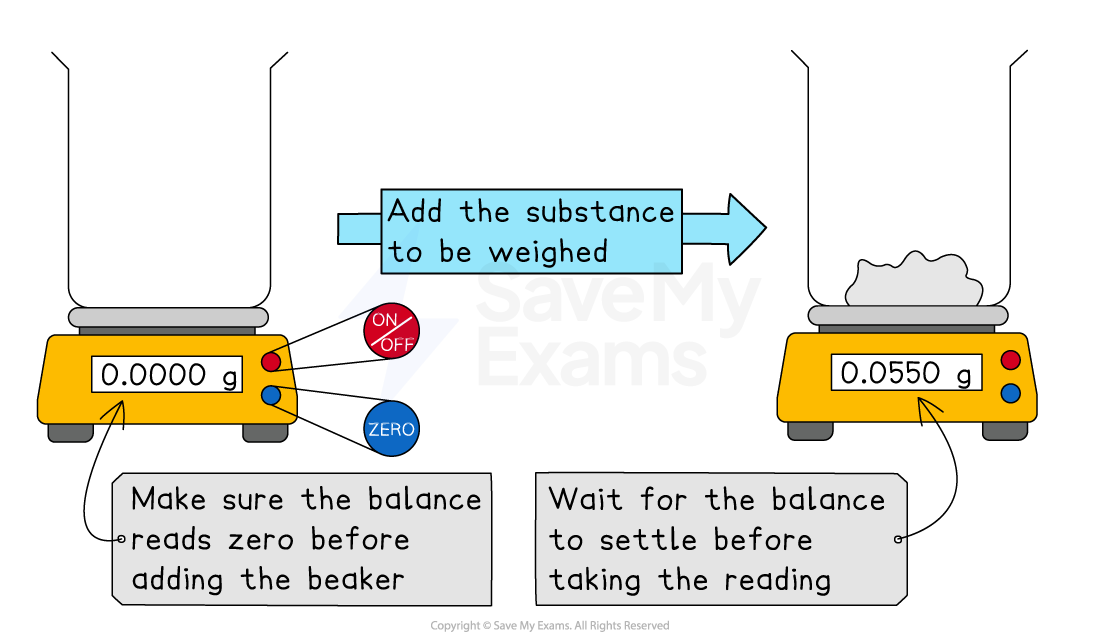
Using a balance
A digital balance is used to accurately measure mass, usually in grams (g)
How to use a balance
Place an empty beaker or weighing boat on the balance
Press the tare (or zero) button to reset the display to zero
This removes the mass of the container
Carefully add the substance to be weighed until you reach the desired mass
Wait for the reading to settle before recording it
Collecting gases
The method used to collect a gas depends on:
Its solubility in water
Its density compared to air
The three main methods for collecting gases are:
Collecting over water
Upward displacement of air
Downward displacement of air
Examiner Tips and Tricks
A gas syringe is also a common and accurate method for collecting any gas
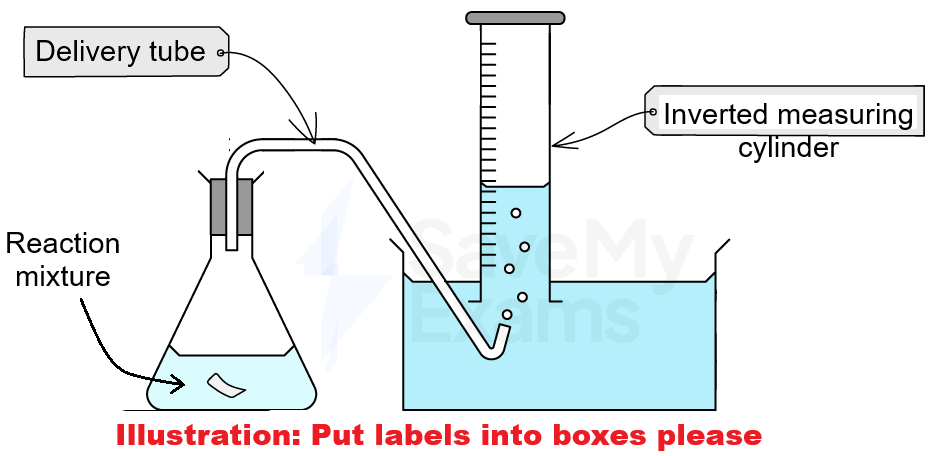
1. Collection over water
This method is used when gases are insoluble or have low solubility in water (e.g., hydrogen)
How it works
As the reaction proceeds, a gas is produced
The gas passes through a delivery tube into an inverted measuring cylinder
The measuring cylinder is filled with water and placed inside a water-filled trough or container
The gas displaces (pushes) the water out of the inverted measuring cylinder
The volume of gas being produced can be measured using this method
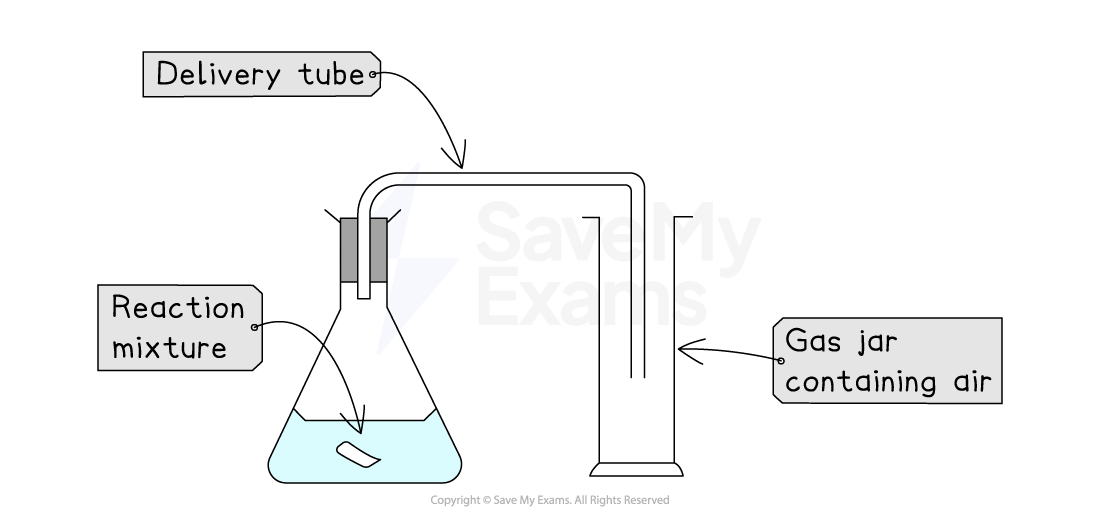
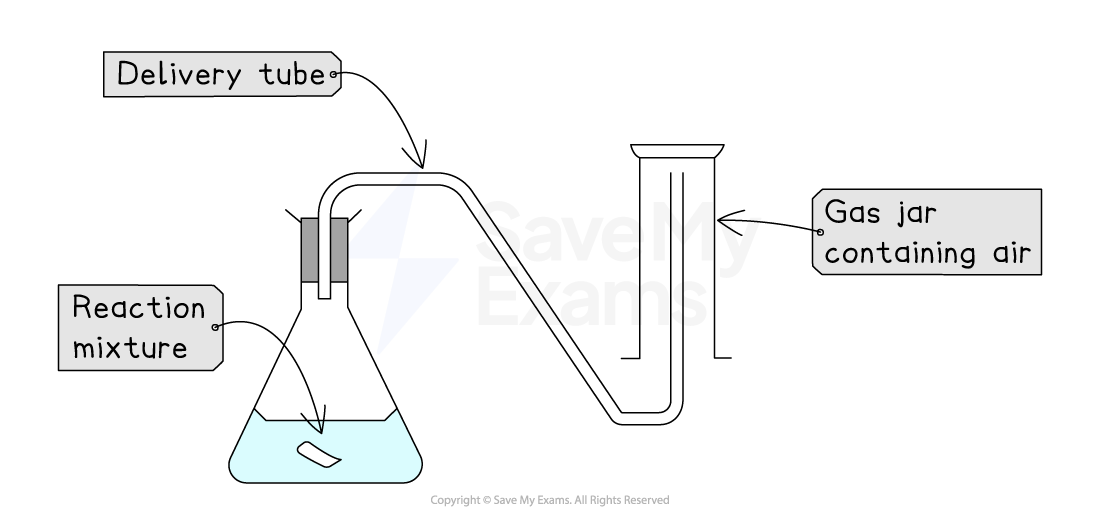
2. Upward displacement of air
This method is used when gases are soluble in water AND denser (heavier) than air (e.g., carbon dioxide)
How it works:
As the reaction proceeds, a gas is produced
The gaseous product passes through a delivery tube into the bottom of an upright gas jar, filled with air
The gaseous product fills the gas jar and pushes the less dense air out of the top of the gas jar
3. Downward displacement of air
This method is used when gases are soluble in water AND less dense (lighter) than air (e.g., ammonia)
How it works:
As the reaction proceeds, a gas is produced
The gaseous product passes through a delivery tube into the top of an inverted gas jar, filled with air
The gaseous product fills the gas jar and pushes the less dense air out of the bottom of the gas jar
Heating
Heating is a common requirement in many chemical experiments, either to start a reaction or to speed it up
The choice of heating method depends on:
What is being heated
The level of control required
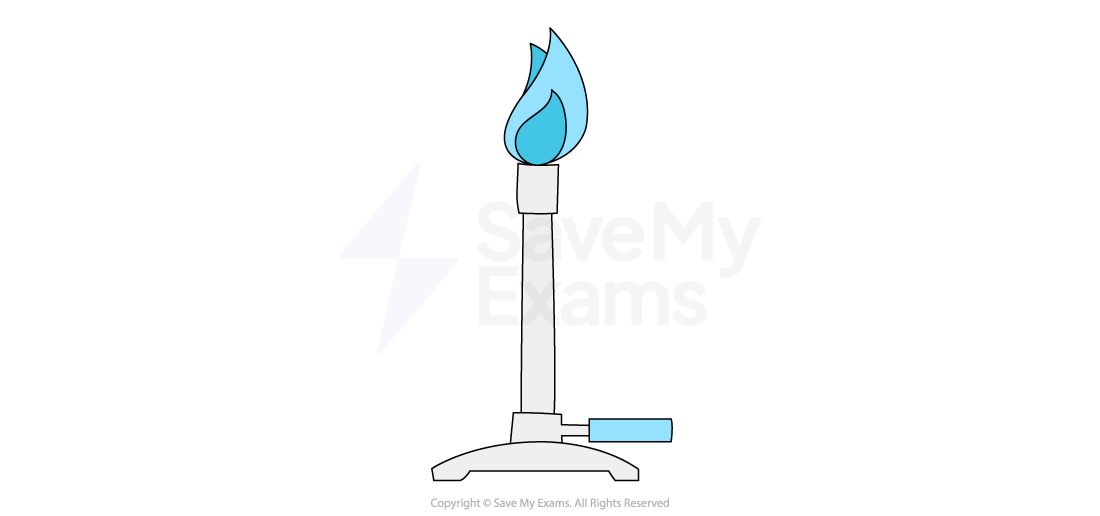
Bunsen burners
A Bunsen burner is the most common piece of heating apparatus in a school lab
It is used for general-purpose heating of substances that are not flammable
For strong, direct heating, you should always use a blue flame, which is hotter and cleaner than the yellow safety flame
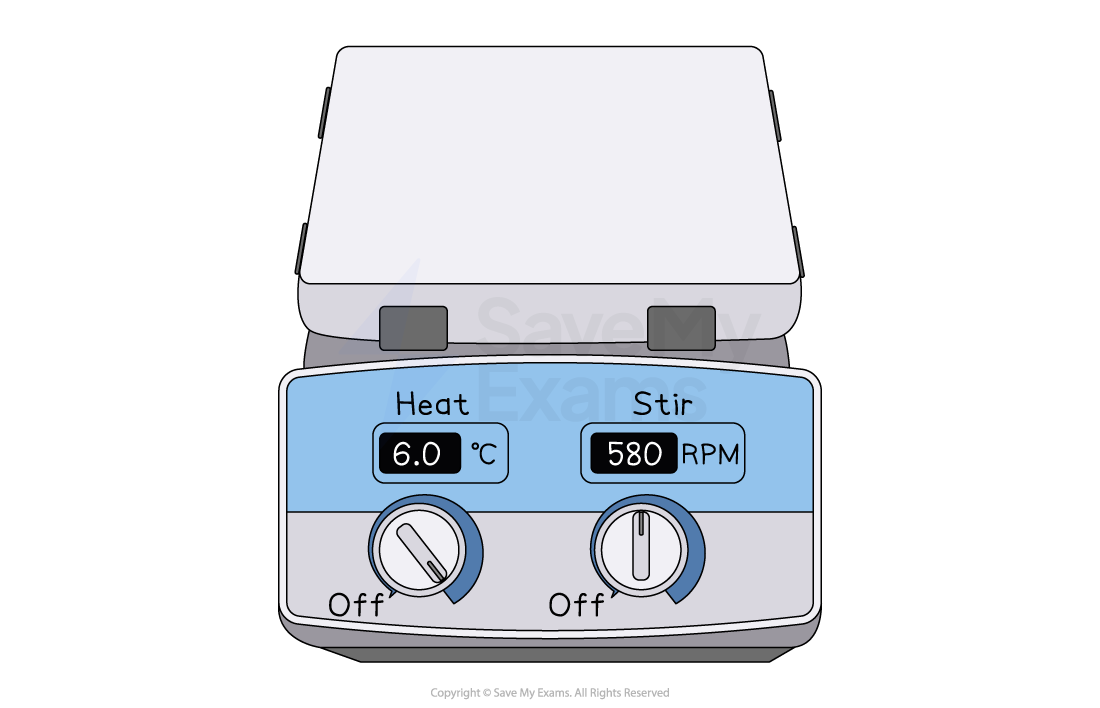
Electric hotplates
An electric hotplate is a safer alternative to a Bunsen burner, particularly when heating flammable liquids like alcohols
Since there is no open flame, the risk of the substance catching fire is eliminated
Hotplates also provide more gentle and easily controllable heating

Unlock more, it's free!
Was this revision note helpful?
