Using Fertilisers (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
NPK fertilisers
Fertilisers are substances which restore essential elements to the soil, helping plants to grow healthily
Plants require several nutrients, but the three most important (or "essential") elements found in fertilisers are:
Nitrogen, N
Phosphorus, P
Potassium, K
Each element helps the plant in a different way
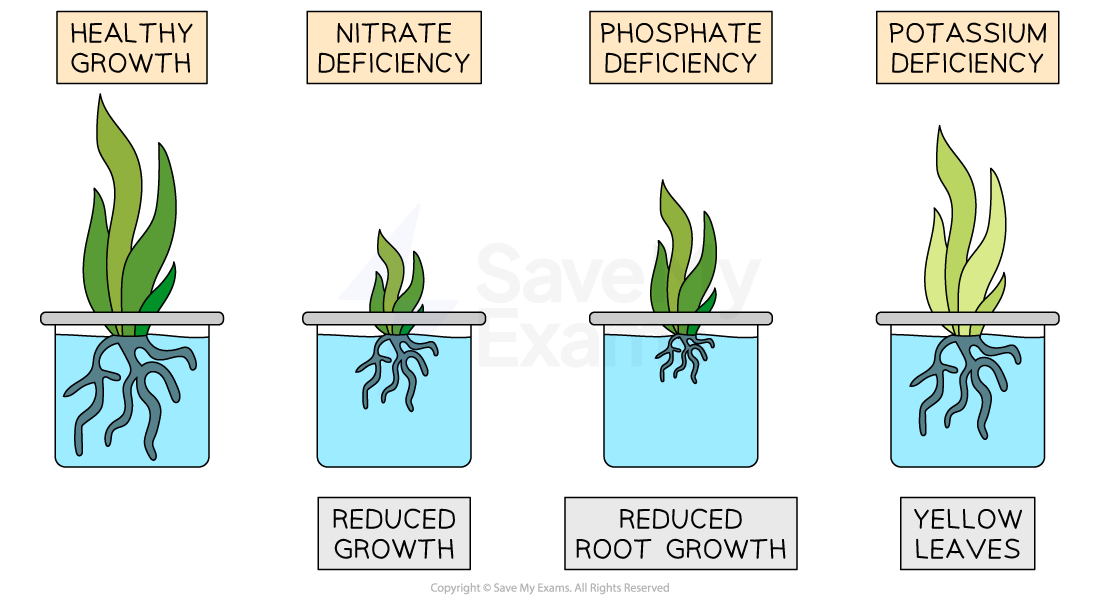
Nitrogen, N
Promotes healthy leaf growth
A nitrogen deficiency leads to reduced overall growth
Phosphorus, P
Promotes healthy root growth
A phosphate deficiency leads to stunted roots
Potassium, K
Promotes the growth of healthy flowers and fruits
A potassium deficiency can lead to yellow leaves
To be effective, fertilisers must be soluble in water so that they can be absorbed by the plant's roots
Nitrogen-containing salts
Nitrogen is a crucial element for plants
Soluble nitrogen-containing salts, used in fertilisers, are made by reacting ammonia with various acids
Ammonia, NH3 (g)
Ammonia is a pungent, colourless gas
It is very soluble in water
When it dissolves, it forms an alkaline solution called ammonia solution
Ammonia solution can be represented by the chemical formula NH4OH (ammonium hydroxide)
Making ammonium salts
Ammonia solution is an alkali
So, it can be neutralised by an acid to produce a soluble salt
These are known as ammonium salts
They are excellent nitrogen-rich fertilisers
The general word equation for these reactions is:
ammonia solution + acid → ammonium salt + water
The key industrial reaction
The production of ammonium nitrate uses both of the key industrial chemicals, ammonia and nitric acid:
ammonia solution + nitric acid → ammonium nitrate + water
NH4OH (aq) + HNO3 (aq) → NH4NO3 (aq) + H2O (l)
Ammonium nitrate supplies nitrogen
The syllabus highlights this as the most important example of making a nitrogen-based fertiliser
Other examples of fertiliser salts
The same neutralisation principle can be used with other acids and bases to make different fertilisers that supply other essential elements
Ammonium phosphate
Phosphoric acid can be used to create a fertiliser that provides both nitrogen and phosphorus:
ammonia solution + phosphoric acid → ammonium phosphate + water
3NH4OH (aq) + H3PO4 (aq) → (NH4)3PO4 (aq) + 3H2O (l)
Ammonium phosphate supplies nitrogen and phosphorus
Potassium nitrate
To create a fertiliser with potassium and nitrogen, a different alkali is needed, such as potassium hydroxide:
potassium hydroxide + nitric acid → potassium nitrate + water
KOH (aq) + HNO3 (aq) → KNO3 (aq) + H2O (l)
Potassium nitrate supplies potassium and nitrogen
Examiner Tips and Tricks
An exam question might give you a list of chemical formulae and ask you to identify which essential elements they provide.
You need to be able to look at a formula and spot the symbols N, P, and K.
NH4NO3 contains nitrogen, N
(NH4)3PO4 contains nitrogen, N, and phosphorus, P
KNO3 contains potassium, K, and nitrogen, N
The most effective fertilisers provide as many of these essential elements as possible.

Unlock more, it's free!
Was this revision note helpful?
