Radiation (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Radioactive decay
Some atoms have an unstable nucleus
These unstable atoms are called radioisotopes.
To become more stable, the nucleus of a radioisotope gives out radiation
This process is called radioactive decay
It involves changes only in the nucleus of the atom
Three different types of radiation that can be emitted are:
Alpha (α)
Beta (β)
Gamma (γ)
What are alpha, beta, and gamma radiation?
Alpha radiation
The symbol for alpha is α
Alpha radiation is a "particle"
An alpha particle is the same as a helium nucleus
This is because they consist of two neutrons and two protons
Beta radiation
The symbol for beta is β
Beta radiation is also a "particle"
Beta particles are fast-moving electrons
They are produced in nuclei when a neutron changes into a proton and an electron
Gamma radiation
The symbol for gamma is γ
Gamma radiation is not a "particle", it is a ray
Gamma rays are high-energy electromagnetic waves

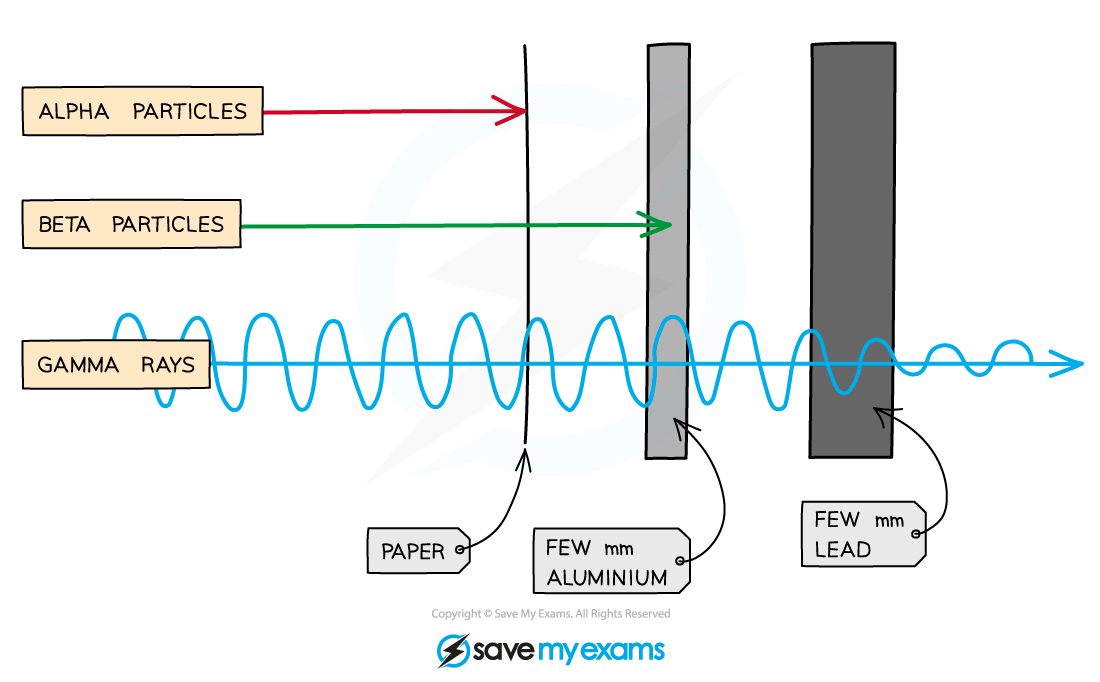
Penetrating power
Penetrating power is the ability of radiation to pass through different materials
Each type of radiation has a different penetrating power
Alpha radiation
Alpha radiation has low penetrating power
It is stopped by a single sheet of paper
Beta radiation
Beta radiation has medium penetrating power
It can pass through paper
But, it is stopped by a thin sheet of aluminium
Gamma radiation
Gamma radiation has high penetrating power
It can pass through paper and aluminium
It is only stopped by thick barriers of lead or concrete
The three types of radiation have different abilities to pass through materials.
Worked Example
Suggest a reason why an alpha-emitting source is unsuitable for monitoring the thickness of cardboard in a factory.
[1]
Answer:
"Alpha radiation has low penetrating power" is not enough to get the mark
This does not explain why an alpha-emitting source is unsuitable for the job
Alpha radiation would be completely stopped by the cardboard, so no radiation would reach the detector [1 mark]
Effect of an electric field
The three types of radiation have different charges
This means that they behave differently in an electric field
Alpha radiation
Alpha radiation has a charge of +2
This positive charge means that it is attracted to the negative plate in an electric field
Beta radiation
Beta radiation has a charge of -1
This negative charge means that it is attracted to the positive plate in an electric field
Gamma radiation
Gamma radiation has a charge of 0
This lack of charge means that it is not deflected and passes straight through

Summary of radiation properties
Property | Alpha (α) | Beta (β) | Gamma (γ) |
|---|---|---|---|
Composition | 2 protons, 2 neutrons | electron | electromagnetic wave |
Charge | +2 | -1 | 0 |
Stopped by... | paper | aluminium | lead / concrete |
Deflection in an electric field | towards the negative plate | towards the positive plate | not deflected |

Unlock more, it's free!
Was this revision note helpful?
