Use of Radioactive Isotopes (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
Using isotopes
Radioisotopes have a wide range of uses in medicine and industry
You need to be able to look at information about an isotope and evaluate its suitability for a particular job
To do this, two key properties should be considered:
The half-life of the isotope
Choosing the right type of radiation
The choice of alpha, beta, or gamma depends on the job
The main consideration is penetrating power
Alpha (α)
Alpha radiation has low penetrating power
It is stopped by paper
It cannot pass through skin
It is only useful if the source is very close to the target and in the open air
A good example of using alpha radiation is inside a smoke detector
Beta (β)
Beta radiation has medium penetrating power
It is stopped by aluminium
It can penetrate skin but not dense bone
It is useful for monitoring the thickness of materials like paper or aluminium foil
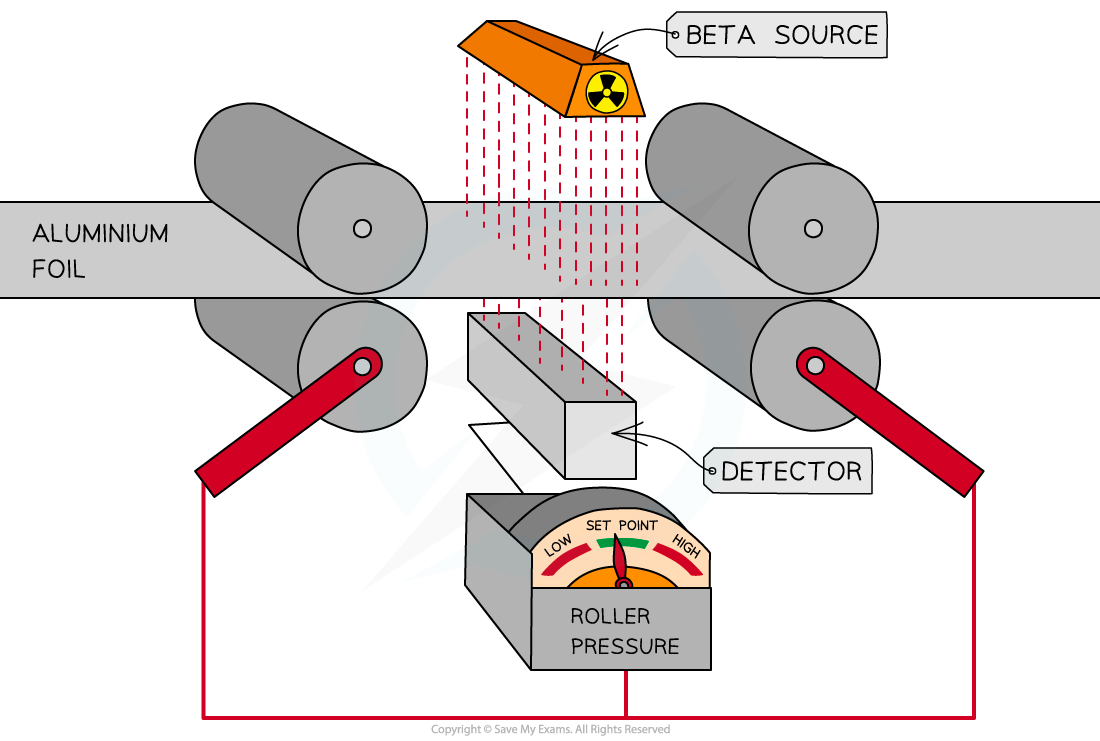
Gamma (γ)
Gamma radiation has the highest penetrating power
It is stopped by thick lead or concrete
It is used for tasks that require the radiation to pass through objects or the human body, such as:
Sterilising equipment
Treating internal tumours
Choosing the right half-life
The choice of half-life depends on how long you need the source to be active
Long half-life (years)
This is needed for jobs where the source must work reliably for a long time without being replaced
Examples include smoke detectors and industrial thickness gauges
Short half-life (hours or days)
This is essential for medical tracers that are put inside the human body
This is because the isotope needs to:
Last long enough for the scan to be completed
But, decay quickly afterwards to minimise the radiation dose to the patient
Worked Example
A doctor is choosing a radioisotope to use as a medical tracer for scanning a patient's internal organs. The isotope will be injected into the patient.
The properties of two available isotopes are shown in the table.
Isotope | Radiation Emitted | Half-life |
|---|---|---|
A | Alpha (α) | 450 years |
B | Gamma (γ) | 6 hours |
Explain which isotope, A or B, is the suitable choice for the medical tracer.
Your answer should include reasons why it is suitable and why the other is unsuitable.
[2]
Answer:
Isotope B is the suitable choice because:
It emits gamma radiation
This is highly penetrating
So, it can pass out of the body and be detected by the scanner
It has a short half-life (6 hours)
This is long enough for the scan to be completed
But, this is short enough for the radiation to decay quickly afterwards
So, the radiation dose for the patient is minimised [1 mark]
Isotope A is unsuitable because:
It emits alpha radiation
This has very low penetrating power
So, it would be stopped by the body's tissues
This means the radiation would be trapped inside the body and not be detected
It has a very long half-life (450 years)
So, it would remain in the patient's body for a dangerously long time [1 mark]

Unlock more, it's free!
Was this revision note helpful?
