Cycloalkanes (SQA National 5 Chemistry): Revision Note
Exam code: X813 75
The cycloalkanes
What are cycloalkanes?
Cycloalkanes are a family of hydrocarbons
For a full table of the first cycloalkanes and their structures, see the Full & shortened structural formulae
Cycloalkanes are a homologous series with four key facts:
1. They are saturated, cyclic hydrocarbons
This means they contain only carbon and hydrogen atoms
All of the carbon-carbon bonds in their structure are single bonds (C-C)
The carbon atoms are joined together to form a ring structure
2. They are represented by a general formula
The general formula for the cycloalkane homologous series is CnH2n
This is the same general formula as the alkenes, which means that cycloalkanes and alkenes with the same number of carbon atoms are isomers.
For example, cyclopropane and propene both have the molecular formula C3H6
3. They are used as fuels and solvents
Like alkanes, they burn well and are used as fuels
Complete combustion of cycloalkanes produces only carbon dioxide and water
They are also effective at dissolving other substances, so they are used as industrial solvents
4. They are insoluble in water
Just like alkanes, cycloalkanes do not mix with water
Names & formulae of cycloalkanes
Like other organic compounds, cycloalkanes are named systematically
There are three skills for cycloalkanes:
Naming cycloalkanes from their structure
Drawing cycloalkanes from their name
Determining the formula of a cycloalkane
The rules for naming and drawing un-branched cycloalkanes are straightforward
Examiner Tips and Tricks
You do not need to know how to name or draw branched cycloalkanes for this course
1. Naming cycloalkanes
The name is made of two parts:
The prefix cyclo- to show it is a ring
The standard alkane name (e.g., propane, butane) to show the number of carbon atoms in the ring
The minimum number of carbon atoms required to form a ring is three
Therefore, the smallest cycloalkane is cyclopropane
Worked Example
Name the cycloalkane shown below.

[1]
Answer:
Ring structure:
It is a ring structure, so the name starts with cyclo-
Number of carbons:
There are 7 carbon atoms in the ring
The prefix for 7 is "hept-"
Carbon-carbon bonds:
All carbon-carbon bonds are single bonds
It is saturated, so the name ends in -ane
Combine the parts:
The full name is cycloheptane [1 mark]
2. Drawing cycloalkanes
You can work backwards from the name to draw the structure of a cycloalkane
"cyclo-" means a ring
The alkane name indicates how many carbon atoms are in the molecule
The rules for drawing cycloalkanes:
Identify the carbon ring
Identify the number of carbons in the ring from the alkane part of the name
For example, "-pentane" means a 5-carbon ring
Add the hydrogens to complete the structure
Make sure every carbon atom has exactly four bonds
Worked Example
Draw the full structural formula for cyclooctane.
[1]
Answer:
The carbon ring:
"cyclo-" means a ring
"oct-" means 8 carbons
"-ane" means single bonds.
So, the full structural formula for cyclooctane is:
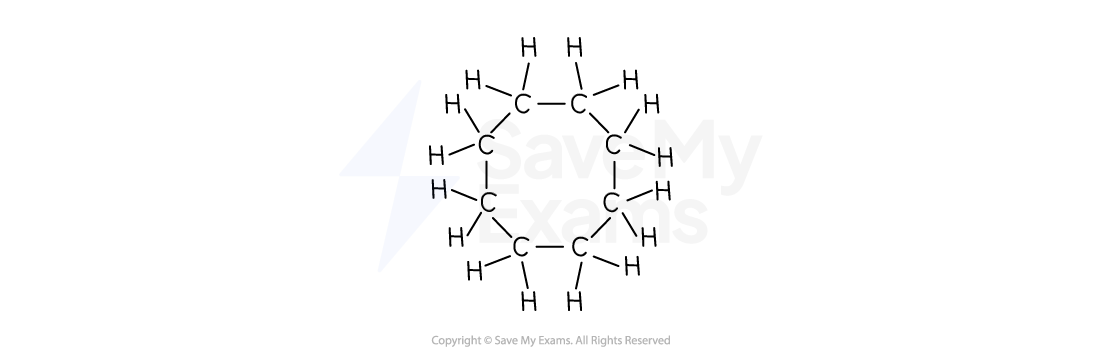
[1 mark]
3. The molecular formula of cycloalkanes
The general formula of cycloalkanes can be used to determine the molecular formula
Worked Example
Give the molecular formula of cycloheptane.
[1]
Answer:
The general formula of a cycloalkane is CnH2n
Hept means that n = 7
So, the formula of cycloheptane is C7H(2 x 7) = C7H14 [1 mark]

Unlock more, it's free!
Was this revision note helpful?
